Innate self recognition by an invariant, rearranged T-cell receptor and its immune consequences
- PMID: 12757612
- PMCID: PMC1782955
- DOI: 10.1046/j.1365-2567.2003.01657.x
Innate self recognition by an invariant, rearranged T-cell receptor and its immune consequences
Abstract
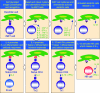
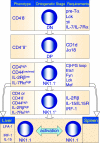
This review attempts to illuminate the glycolipid antigen presentation properties of CD1d, how CD1d controls the function of natural T (iNKT) cells and how CD1d and iNKT cells interact to jump-start the immune system. It is postulated that the CD1d-iNKT cell system functions as a sensor, sensing alterations in cellular lipid content by virtue of its affinity for such ligands. The presentation of a neo-self glycolipid, presumably by infectious assault of antigen-presenting cells, activates iNKT cells, which promptly release pro-inflammatory and anti-inflammatory cytokines and jump-start the immune system.
Figures


Similar articles
-
CD1d and natural T cells: how their properties jump-start the immune system.Cell Mol Life Sci. 2001 Mar;58(3):442-69. doi: 10.1007/PL00000869. Cell Mol Life Sci. 2001. PMID: 11315191 Free PMC article. Review.
-
CD1d-restricted glycolipid antigens: presentation principles, recognition logic and functional consequences.Expert Rev Mol Med. 2008 Jul 7;10:e20. doi: 10.1017/S1462399408000732. Expert Rev Mol Med. 2008. PMID: 18601810 Review.
-
Therapeutic potential of CD1d-restricted invariant natural killer T cell-based treatment for autoimmune diseases.Int Rev Immunol. 2007 Jan-Apr;26(1-2):73-94. doi: 10.1080/08830180601070252. Int Rev Immunol. 2007. PMID: 17454265 Review.
-
Characterization and functional analysis of mouse invariant natural T (iNKT) cells.Curr Protoc Immunol. 2006 Jul;Chapter 14:14.13.1-14.13.27. doi: 10.1002/0471142735.im1413s73. Curr Protoc Immunol. 2006. PMID: 18432968
-
Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells.J Immunol. 2008 May 15;180(10):6452-6. doi: 10.4049/jimmunol.180.10.6452. J Immunol. 2008. PMID: 18453560
Cited by
-
Beta-glycoglycosphingolipid-induced alterations of the STAT signaling pathways are dependent on CD1d and the lipid raft protein flotillin-2.Am J Pathol. 2009 Apr;174(4):1390-9. doi: 10.2353/ajpath.2009.080841. Epub 2009 Feb 26. Am J Pathol. 2009. PMID: 19246642 Free PMC article.
-
Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny.Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5114-9. doi: 10.1073/pnas.0408449102. Epub 2005 Mar 25. Proc Natl Acad Sci U S A. 2005. PMID: 15792999 Free PMC article.
-
Alpha versus beta: are we on the way to resolve the mystery as to which is the endogenous ligand for natural killer T cells?Clin Exp Immunol. 2009 Dec;158(3):300-7. doi: 10.1111/j.1365-2249.2009.04030.x. Epub 2009 Sep 30. Clin Exp Immunol. 2009. PMID: 19793337 Free PMC article.
References
-
- Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nature Rev Immunol. 2001;1:177–86. - PubMed
-
- Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells – conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–71. - PubMed
-
- Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nature Med. 2001;7:1052–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

