Soluble FcgammaRIIa inhibits rheumatoid factor binding to immune complexes
- PMID: 12757620
- PMCID: PMC1782965
- DOI: 10.1046/j.1365-2567.2003.01652.x
Soluble FcgammaRIIa inhibits rheumatoid factor binding to immune complexes
Abstract
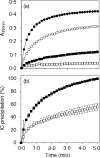
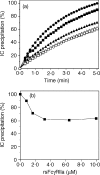
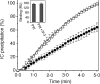
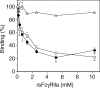
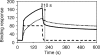
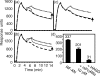
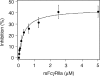
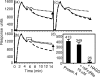
Soluble low-affinity receptors for IgG are known to inhibit immune complex (IC)-mediated inflammation, and expression by leukocytes is elevated in several inflammatory diseases. Immunoglobulin M (IgM) rheumatoid factors (RF), anti-Fc autoantibodies, are found in autoimmune diseases, such as rheumatoid arthritis (RA), as well as in normal immune responses. This study demonstrated that soluble FcgammaRIIa inhibits the interaction of rheumatoid factors with ICs. The recombinant soluble low-affinity FcgammaR, rsFcgammaRIIa, partially inhibited (30-70%) the rate of precipitation of soluble ICs by RF-positive RA sera. This required the normal interaction of FcgammaRIIa with Fc as the effect could be abrogated with the Fab fragment of the blocking mAb IV-3. Furthermore, rsFcgammaRIIa partially inhibited (40%) the binding of a monoclonal IgM RF (RF-AN) to an IC formed by IgG2 antibody binding to an antigen-coated biosensor chip. Since RF-AN has been characterized by crystallography to bind to the CH2/CH3 interface of the IgG-Fc, and leukocyte FcgammaRIIa binds to a distinct site centred on the lower hinge, this inhibition is uncompetitive. Some inhibition (15%) of staphylococcal protein A binding to IC was also observed. As soluble FcgammaRIIa disrupts Fc:Fc interactions in IgG-ICs, we propose that this alteration of the IC also reduces the accessibility of Fc portions in the IC, resulting in the partial inhibition of ligands, particularly IgM RF, which bind Fc. We propose that the high concentrations of soluble FcgammaR found during inflammation can affect the properties of ICs and their interaction with the immune system.
Figures
References
-
- Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. - PubMed
-
- Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 1994;265:1095–8. - PubMed
-
- Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–60. - PubMed
-
- Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–92. - PubMed
-
- Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc (gamma) RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

