Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes
- PMID: 12757624
- PMCID: PMC1782969
- DOI: 10.1046/j.1365-2567.2003.01643.x
Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes
Abstract
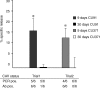
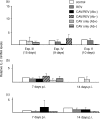
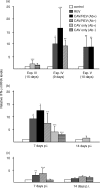
Infection with chicken anaemia virus (CAV), a circovirus, can result in immunosuppression and subsequent increased susceptibility to secondary infections. This is the first report of impairment of pathogen-specific cytotoxic T lymphocytes (CTL) after natural and experimental infection of chickens with CAV and Marek's disease virus (MDV) or reticuloendotheliosis virus (REV). MDV- and REV-specific CTL were generated at 7 days post infection by 9-30-day-old-chickens that were positive for maternal antibodies to CAV at 9-17 days of age. Replication of CAV could not be demonstrated in these chickens using quantitative real-time polymerase chain reaction (PCR) and reverse transcriptase (RT)-PCR assays. In contrast, REV-specific CTL failed to develop when chickens negative for maternal antibodies at 9-17 days of age were infected. Infection with CAV at 45 days of age after CAV maternal antibodies had waned also caused a decreased REV-specific CTL response. In these chickens increased levels of CAV DNA of up to 107 copy numbers per micro g DNA and increased relative transcript levels of CAV by up to a factor of 106 were detected by quantitative real-time PCR and RT-PCR. Interleukin (IL)-1beta and IL-2 mRNA levels were not significantly affected by CAV infection at 7 or 14 days p.i. Similar assays for interferon-gamma (IFN-gamma) transcripts demonstrated a 10-fold increase in IFN-gamma mRNA levels at 7 days post infection following REV or REV + CAV infection, while CAV alone caused a two- to fourfold increase. These results show a strong link between CAV antibody status, CAV replication, and the ability to generate REV-specific CTL. It is likely that the immunosuppressive effects of subclinical infection have previously been underestimated.
Figures

References
-
- Pringle CR. Virus taxonomy at the XIth International congress of Virology, Sydney, Australia 1999. Arch Virol. 1999;144:2065–9. - PubMed
-
- Noteborn MH, Kranenburg O, Zantema A, Koch G, de Boer GF, van der Eb AJ. Transcription of the chicken anemia virus (CAV) genome and synthesis of its 52-kDa protein. Gene. 1992;118:267–71. - PubMed
-
- Phenix KV, Meehan BM, Todd D, McNulty MS. Transcriptional analysis and genome expression of chicken anaemia virus. J Gen Virol. 1994;75:905–9. - PubMed
-
- Koch G, van Roozelaar DJ, Verschueren CA, van der Eb AJ, Noteborn MH. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine. 1995;13:763–70. - PubMed
-
- Noteborn MH, Verschueren CA, Koch G, Van der Eb AJ. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J Gen Virol. 1998;79:3073–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

