Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells
- PMID: 12757626
- PMCID: PMC1782954
- DOI: 10.1046/j.1365-2567.2003.01656.x
Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells
Abstract
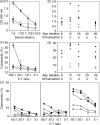
The tumour-associated antigen mucin 1 (MUC1) is a multifunctional protein involved in protection of mucous membranes, signal transduction, and modulation of the immune system. More than 70% of cancers overexpress MUC1, making MUC1 a potential target for immunotherapy. In the present study, MUC1 transgenic mice were crossed with syngeneic strains that express the polyomavirus middle-T oncogene (PyMT) driven by the mouse mammary tumour virus promoter long-terminal repeat (MMTV-LTR). The resultant breed (MMT mice) developed spontaneous MUC1-expressing mammary carcinomas with 100% penetrance at 8-15 weeks of age. As found in human breast cancer, the mammary carcinoma in MMT mice arose in multiple stages. Immunization with fusions of dendritic cells and MUC1-positive tumour cells (FC/MUC1) induced MUC1-specific immune responses that blocked or delayed the development of spontaneous breast carcinomas. In contrast, there was no delay of tumour development in MMT mice immunized with irradiated MC38/MUC1 tumour cells. The efficacy of fusion cells was closely correlated with the timing of initial immunization. Immunization with FC/MUC1 initiated in MMT mice at < 1, 1-2 and 2-3 months of age rendered 33, 5 and 0% of mice free of tumour, respectively, up to 6 months. Whereas mice immunized in the later stage of tumour development succumbed to their disease, immunization resulted in control of tumour progression and prolongation of life. These results indicate that immunization with FC/MUC1 can generate an anti-MUC1 response that is sufficient to delay the development of spontaneous mammary carcinomas and control tumour progression in MMT mice.
Figures




Similar articles
-
The Tat-conjugated N-terminal region of mucin antigen 1 (MUC1) induces protective immunity against MUC1-expressing tumours.Clin Exp Immunol. 2009 Nov;158(2):174-85. doi: 10.1111/j.1365-2249.2009.03997.x. Epub 2009 Jul 17. Clin Exp Immunol. 2009. PMID: 19737144 Free PMC article.
-
Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells.J Immunol. 2003 Feb 15;170(4):1980-6. doi: 10.4049/jimmunol.170.4.1980. J Immunol. 2003. PMID: 12574367
-
Selection and characterization of MUC1-specific CD8+ T cells from MUC1 transgenic mice immunized with dendritic-carcinoma fusion cells.Immunology. 2000 Nov;101(3):316-24. doi: 10.1046/j.1365-2567.2000.00101.x. Immunology. 2000. PMID: 11106934 Free PMC article.
-
Tecemotide: an antigen-specific cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3383-93. doi: 10.4161/hv.29836. Hum Vaccin Immunother. 2014. PMID: 25483673 Free PMC article. Review.
-
Targeted Immunotherapy Designed to Treat MUC1-Expressing Solid Tumour.Curr Top Microbiol Immunol. 2017;405:79-97. doi: 10.1007/82_2015_429. Curr Top Microbiol Immunol. 2017. PMID: 25702159 Review.
Cited by
-
Targeted imaging of breast tumor progression and therapeutic response in a human uMUC-1 expressing transgenic mouse model.Int J Cancer. 2013 Apr 15;132(8):1860-7. doi: 10.1002/ijc.27872. Epub 2012 Oct 25. Int J Cancer. 2013. PMID: 23015160 Free PMC article.
-
The Tat-conjugated N-terminal region of mucin antigen 1 (MUC1) induces protective immunity against MUC1-expressing tumours.Clin Exp Immunol. 2009 Nov;158(2):174-85. doi: 10.1111/j.1365-2249.2009.03997.x. Epub 2009 Jul 17. Clin Exp Immunol. 2009. PMID: 19737144 Free PMC article.
-
Combination of pioglitazone and dendritic cell to optimize efficacy of immune cell therapy in CT26 tumor models.Bioimpacts. 2023;13(4):333-346. doi: 10.34172/bi.2022.24209. Epub 2022 Aug 9. Bioimpacts. 2023. PMID: 37645031 Free PMC article.
-
Antigen-specific polyclonal cytotoxic T lymphocytes induced by fusions of dendritic cells and tumor cells.J Biomed Biotechnol. 2010;2010:752381. doi: 10.1155/2010/752381. Epub 2010 Apr 7. J Biomed Biotechnol. 2010. PMID: 20379390 Free PMC article.
-
A multi-functional role of interferon regulatory factor-8 in solid tumor and myeloid cell biology.Immunol Res. 2010 Mar;46(1-3):59-71. doi: 10.1007/s12026-009-8125-6. Immunol Res. 2010. PMID: 19756408 Review.
References
-
- Hellstrom KE, Hellstrom I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. - PubMed
-
- Rosato FE. Active specific immunotherapy of human solid tumors. Ann N Y Acad Sci. 1976;277:332–8. - PubMed
-
- Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IF, Bast RC, Jr, Finn OJ. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res. 1991;51:2908–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

