Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium
- PMID: 12759241
- PMCID: PMC1868120
- DOI: 10.1016/S0002-9440(10)64318-0
Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium
Abstract
Matrilysin (matrix metalloproteinase-7) is highly expressed in lungs of patients with pulmonary fibrosis and other conditions associated with airway and alveolar injury. Although matrilysin is required for closure of epithelial wounds ex vivo, the mechanism of its action in repair is unknown. We demonstrate that matrilysin mediates shedding of E-cadherin ectodomain from injured lung epithelium both in vitro and in vivo. In alveolar-like epithelial cells, transfection of activated matrilysin resulted in shedding of E-cadherin and accelerated cell migration. In vivo, matrilysin co-localized with E-cadherin at the basolateral surfaces of migrating tracheal epithelium, and the reorganization of cell-cell junctions seen in wild-type injured tissue was absent in matrilysin-null samples. E-cadherin ectodomain was shed into the bronchoalveolar lavage fluid of bleomycin-injured wild-type mice, but was not shed in matrilysin-null mice. These findings identify E-cadherin as a novel substrate for matrilysin and indicate that shedding of E-cadherin ectodomain is required for epithelial repair.
Figures




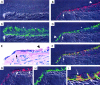
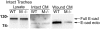
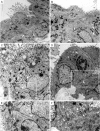

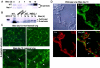
References
-
- Laitinen LA, Heino M, Laitinin A, Kava T, Haahtela T: Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 1985, 131:599-606 - PubMed
-
- Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB: Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis 1989, 140:1745-1753 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

