Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis
- PMID: 12759248
- PMCID: PMC1868139
- DOI: 10.1016/S0002-9440(10)64325-8
Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis
Abstract
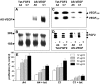
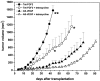
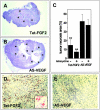
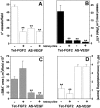
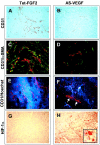
Tumors express more than a single angiogenic growth factor. To investigate the relative impact of fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) on tumor growth and neovascularization, we generated tumor cell transfectants differing for VEGF and/or FGF-2 expression. Human endometrial adenocarcinoma HEC-1-B-derived Tet-FGF-2 cells that express FGF-2 under the control of the tetracycline-responsive promoter (Tet-off system) were further transfected with a VEGF(121) anti-sense (AS-VEGF) cDNA. Next, Tet-FGF-2 and AS-VEGF/Tet-FGF-2 cells were transplanted subcutaneously in nude mice that received tetracycline or not in the drinking water. Simultaneous expression of FGF-2 and VEGF in Tet-FGF-2 cells resulted in fast-growing lesions characterized by high blood vessel density, patency and permeability, and limited necrosis. Blood vessels were highly heterogeneous in size and frequently associated with pericytes. Inhibition of FGF-2 production by tetracycline caused a significant decrease in tumor burden paralleled by a decrease in blood vessel density and size. AS-VEGF expression resulted in a similar reduction in blood vessel density associated with a significant decrease in pericyte organization, vascular patency, and permeability. The consequent decrease in tumor burden was paralleled by increased tumor hypoxia and necrosis. A limited additional inhibitory effect was exerted by simultaneous down-regulation of FGF-2 and VEGF expression. These findings demonstrate that FGF-2 and VEGF stimulate vascularization synergistically but with distinctive effects on vessel functionality and tumor survival. Blockade of either one of the two growth factors results in a decrease in blood vessel density and, consequently, in tumor burden. However, inhibition of the expression of VEGF, but not of FGF-2, affects also vessel maturation and functionality, leading to tumor hypoxia and necrosis. Our experimental model represents an unique tool to investigate anti-neoplastic therapies in different angiogenic environments.
Figures

References
-
- Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 - PubMed
-
- Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 - PubMed
-
- Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL: Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 1997, 57:963-969 - PubMed
-
- Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M: Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 1984, 223:1296-1299 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

