A murine model to study leukocyte rolling and intravascular trafficking in lung microvessels
- PMID: 12759257
- PMCID: PMC1868130
- DOI: 10.1016/S0002-9440(10)64334-9
A murine model to study leukocyte rolling and intravascular trafficking in lung microvessels
Abstract
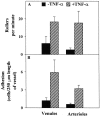
The cascade of leukocyte interactions under conditions of blood flow is well established in the systemic microcirculation, but not in lung microcirculation. We have developed a murine model to study lung microcirculation by transplanting lung tissue into dorsal skin-fold window chambers in nude mice and examining the ability of leukocytes to traffic within revascularized lung microvessels by intravital microscopy. The revascularized lung allograft demonstrated a network of arterioles, capillaries, and postcapillary venules with continuous blood flow. Stimulation of the lung allograft with TNF-alpha induced leukocyte rolling and adhesion in both arterioles and venules. Treatment with function-blocking anti-selectin mAb revealed that P- and L-selectin are the predominant rolling receptors in the lung microvessels, with E-selectin strengthening P-selectin-dependent interactions. Intravital microscopic studies also demonstrated that during their transit in capillaries, some leukocytes undergo shape change and continue to roll as elongated cells in postcapillary venules. Furthermore, the revascularized microvessels demonstrated the ability to undergo vasoconstriction in response to superfusion with endothelin-1. Overall, these studies demonstrate that the revascularized lung allograft is responsive to various external stimuli such as cytokines and vaso-active mediators and serves as a model to evaluate the interaction of leukocytes with the vascular endothelium in the lung microcirculation under acute as well as chronic experimental conditions.
Figures








References
-
- Kuebler WM, Kuhnle GEH, Groh J, Goetz AE: Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol 1994, 76:65-71 - PubMed
-
- Kuhnle GEH, Leipfinger FH, Goetz AE: Measurement of microhemodynamics in the ventilated rabbit lung by intravital fluorescence microscopy. J Appl Physiol 1993, 74:1462-1471 - PubMed
-
- Davis MJ, Gilmore JP, Joyner WL: Responses of pulmonary allograft and cheek pouch arterioles in the hamster to alterations in extravascular pressure in different oxygen environments. Circ Res 1981, 49:133-140 - PubMed
-
- Davis MJ, Joyner WL, Gilmore JP: Microvascular pressure distribution and responses of pulmonary allografts and cheek pouch arterioles in the hamster to oxygen. Circ Res 1981, 49:125-132 - PubMed
-
- Shepard J, Joyner WL, Gilmore JP: Hypoxia does not alter angiotensin converting enzyme activity in hamster pulmonary microvessels. Circ Res 1987, 61:228-235 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

