Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation
- PMID: 12759258
- PMCID: PMC1868132
- DOI: 10.1016/S0002-9440(10)64335-0
Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation
Abstract
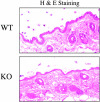
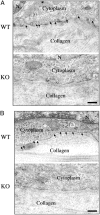
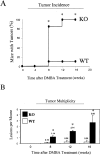
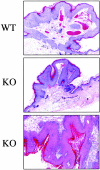
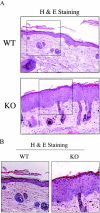
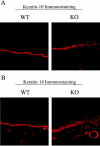
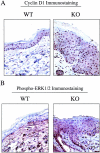
Caveolin-1 is the principal protein component of caveolae membrane domains, which are located at the cell surface in most cell types. Evidence has accumulated suggesting that caveolin-1 may function as a suppressor of cell transformation in cultured cells. The human CAV-1 gene is located at a putative tumor suppressor locus (7q31.1/D7S522) and a known fragile site (FRA7G) that is deleted in a variety of epithelial-derived tumors. Mechanistically, caveolin-1 is known to function as a negative regulator of the Ras-p42/44 MAP kinase cascade and as a transcriptional repressor of cyclin D1, possibly explaining its transformation suppressor activity in cultured cells. However, it remains unknown whether caveolin-1 functions as a tumor suppressor gene in vivo. Here, we examine the tumor suppressor function of caveolin-1 using Cav-1 (-/-) null mice as a model system. Cav-1 null mice and their wild-type counterparts were subjected to carcinogen-induced skin tumorigenesis, using 7,12-dimethylbenzanthracene (DMBA). Mice were monitored weekly for the development of tumors. We demonstrate that Cav-1 null mice are dramatically more susceptible to carcinogen-induced tumorigenesis, as they develop skin tumors at an increased rate. After 16 weeks of DMBA-treatment, Cav-1 null mice showed a 10-fold increase in tumor incidence, a 15-fold increase in tumor number per mouse (multiplicity), and a 35-fold increase in tumor area per mouse, as compared with wild-type littermate mice. Moreover, before the development of tumors, DMBA-treatment induced severe epidermal hyperplasia in Cav-1 null mice. Both the basal cell layer and the suprabasal cell layers were expanded in treated Cav-1 null mice, as evidenced by immunostaining with cell-type specific differentiation markers (keratin-10 and keratin-14). In addition, cyclin D1 and phospho-ERK1/2 levels were up-regulated during epidermal hyperplasia, suggesting a possible mechanism for the increased susceptibility of Cav-1 null mice to tumorigenesis. However, the skin of untreated Cav-1 null mice appeared normal, without any evidence of epidermal hyperplasia, despite the fact that Cav-1 null keratinocytes failed to express caveolin-1 and showed a complete ablation of caveolae formation. Thus, Cav-1 null mice require an appropriate oncogenic stimulus, such as DMBA treatment, to reveal their increased susceptibility toward epidermal hyperplasia and skin tumor formation. Our results provide the first genetic evidence that caveolin-1 indeed functions as a tumor suppressor gene in vivo.
Figures




References
-
- Razani B, Woodman SE, Lisanti MP: Caveolae: from cell biology to animal physiology. Pharmacol Rev 2002, 54:431-467 - PubMed
-
- Glenney JR, Jr: Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem 1989, 264:20163-20166 - PubMed
-
- Engelman JA, Wycoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP: Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem 1997, 272:16374-16381 - PubMed
-
- Park DS, Razani B, Lasorella A, Schreiber-Agus N, Pestell RG, Iavarone A, Lisanti MP: Evidence that Myc isoforms transcriptionally repress caveolin-1 gene expression via an INR-dependent mechanism. Biochemistry 2001, 40:3354-3362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

