Structural and functional consequences of cleavage of human secretory and human serum immunoglobulin A1 by proteinases from Proteus mirabilis and Neisseria meningitidis
- PMID: 12761118
- PMCID: PMC155769
- DOI: 10.1128/IAI.71.6.3349-3356.2003
Structural and functional consequences of cleavage of human secretory and human serum immunoglobulin A1 by proteinases from Proteus mirabilis and Neisseria meningitidis
Abstract
The cleavage of human serum monomeric immunoglobulin A1 (IgA1) and human secretory IgA1 (S-IgA1) by IgA1 proteinase of Neisseria meningitidis and cleavage by the proteinase from Proteus mirabilis have been compared. For serum IgA1, both proteinases cleaved only the alpha chain. N. meningitidis proteinase cleaved only in the hinge. P. mirabilis proteinase sequentially removed the tailpiece, the CH3 domain, and the CH2 domain. The cleavage of S-IgA1 by N. meningitidis proteinase occurred only in the hinge and was as rapid as that of serum IgA1. P. mirabilis proteinase predominantly cleaved the secretory component (SC) of S-IgA1. The SC of S-IgA1, whether cleaved or not, appeared to protect the alpha1 chain. Purified Fc fragment derived from the cleavage of serum IgA1 by N. meningitidis proteinase stimulated a respiratory burst in neutrophils through Fcalpha receptors, whereas the (Fcalpha1)(2)-SC fragment from digested S-IgA1 did not. The loss of the tailpiece from serum IgA1 treated with P. mirabilis proteinase had little effect, but the loss of the CH3 domain was concurrent with a rapid loss in the ability to bind to Fcalpha receptors. S-IgA1 treated with P. mirabilis proteinase under the same conditions retained the ability to bind to Fcalpha receptors. The results are consistent with the Fcalpha receptor binding site being at the CH2-CH3 interface. These data shed further light on the structure of S-IgA1 and indicate that the binding site for the Fcalpha receptor in S-IgA is protected by SC, thus prolonging its ability to activate phagocytic cells at the mucosal surface.
Figures
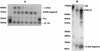





References
-
- Boehm, M. K., J. M. Woof, M. A. Kerr, and S. J. Perkins. 1999. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron solution scattering and homology modelling. J. Mol. Biol. 286:1421-1447. - PubMed
-
- Brooks, G. F., C. J. Lammel, M. S. Blake, B. Kusecek, and M. Achtman. 1992. Antibodies against IgA protease are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J. Infect. Dis. 166:1316-1321. - PubMed
-
- Devenyi, A. G., A. G. Plaut, F. J. Grundy, and A. Wright. 1993. Post-infectious human serum antibodies inhibit IgA1 proteinases by interaction with the cleavage site specificity determinant. Mol. Immunol. 30:1243-1248. - PubMed
-
- Gilbert, J. V., A. G. Plaut, B. Longmaid, and M. E. Lamm. 1983. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol. Immunol. 20:1039-1049. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

