Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection
- PMID: 12761147
- PMCID: PMC155743
- DOI: 10.1128/IAI.71.6.3607-3613.2003
Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection
Abstract
Proteus mirabilis, a common cause of nosocomial and catheter-associated urinary tract infection, colonizes the bladder and ascends the ureters to the proximal tubules of the kidneys, leading to the development of acute pyelonephritis. P. mirabilis is capable of swarming, a form of multicellular behavior in which bacteria differentiate from the short rod typical of members of the family Enterobacteriaceae, termed the swimmer cell, into hyperflagellated elongated bacteria capable of rapid and coordinated population migration across surfaces, called the swarmer cell. There has been considerable debate as to which morphotype predominates during urinary tract infection. P. mirabilis(pBAC001), which expresses green fluorescent protein in both swimming and swarming morphotypes, was constructed to quantify the prevalence of each morphotype in ascending urinary tract infection. Transurethral inoculation of P. mirabilis(pBAC001) resulted in ascending urinary tract infection and kidney pathology in mice examined at both 2 and 4 days postinoculation. Using confocal microscopy, we were able to investigate the morphotypes of the bacteria in the urinary tract. Of 5,087 bacteria measured in bladders, ureters, and kidneys, only 7 (0.14%) were identified as swarmers. MR/P fimbria expression, which correlates with the swimmer phenotype, is prevalent on bacteria in the ureters and bladder. We conclude that, by far, the predominant morphotype present in the urinary tract during ascending infection is the short rod-the swimmer cell.
Figures
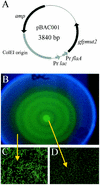
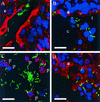
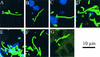
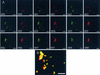
References
-
- Allison, C., L. Emody, N. Coleman, and C. Hughes. 1994. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169:1155-1158. - PubMed
-
- Allison, C., and C. Hughes. 1991. Closely linked genetic loci required for swarm cell differentiation and multicellular migration by Proteus mirabilis. Mol. Microbiol. 5:1975-1982. - PubMed
-
- Allison, C., H.-C. Lai, D. Gygi, and C. Hughes. 1993. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol. Microbiol. 8:53-60. - PubMed
-
- Allison, C., H.-C. Lai, and C. Hughes. 1992. Coordinate expression of virulence genes during swarming cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

