Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles
- PMID: 12761383
- PMCID: PMC165865
- DOI: 10.1073/pnas.0832180100
Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles
Abstract
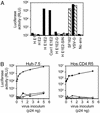
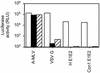
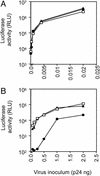
HIV pseudotypes bearing native hepatitis C virus (HCV) glycoproteins (strain H and Con1) are infectious for the human hepatoma cell lines Huh-7 and PLC/PR5. Infectivity depends on coexpression of both E1 and E2 glycoproteins, is pH-dependent, and can be neutralized by mAbs mapping to amino acids 412-447 within E2. Cell-surface expression of one or all of the candidate receptor molecules (CD81, low-density lipoprotein receptor, scavenger receptor class B type 1, and dendritic cell-specific intercellular adhesion molecule 3 grabbing nonintegrin) failed to confer permissivity to HIV-HCV pseudotype infection. However, HIV-HCV pseudotype infectivity was inhibited by a recombinant soluble form of CD81 and a mAb specific for CD81, suggesting that CD81 may be a component of a receptor complex.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

