An electronic effect on protein structure
- PMID: 12761389
- PMCID: PMC2323894
- DOI: 10.1110/ps.0241903
An electronic effect on protein structure
Abstract
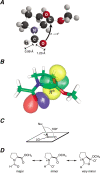
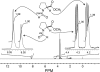
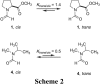
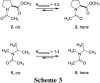
The well-known preference of the peptide bond for the trans conformation has been attributed to steric effects. Here, we show that a proline residue with an N-formyl group (H(i-1)-C'(i-1)=O(i-1)), in which H(i-1) presents less steric hindrance than does O(i-1), likewise prefers a trans conformation. Thus, the preference of the peptide bond for the trans conformation cannot be explained by steric effects alone. Rather, an n --> pi* interaction between the oxygen of the peptide bond (O(i-1)), and the subsequent carbonyl carbon in the polypeptide chain (C'(i)) also contributes to this preference. The O(i-1) and C'(i) distance and O(i-1).C'(i)=O(i) angle are especially favorable for such an n --> pi* interaction in a polyproline II helix. We propose that this electronic effect provides substantial stabilization to this and other elements of protein structure.
Figures


Similar articles
-
Impact of cis-proline analogs on peptide conformation.Biopolymers. 2006 Apr 5;81(5):392-406. doi: 10.1002/bip.20431. Biopolymers. 2006. PMID: 16358327
-
An examination of the steric effects of 5-tert-butylproline on the conformation of polyproline and the cooperative nature of type II to type I helical interconversion.Biopolymers. 2000 Mar;53(3):249-56. doi: 10.1002/(SICI)1097-0282(200003)53:3<249::AID-BIP4>3.0.CO;2-J. Biopolymers. 2000. PMID: 10679629
-
Steric and electronic interactions controlling the cis/trans isomer equilibrium at X-Pro tertiary amide motifs in solution.Biopolymers. 2014 Jan;101(1):66-77. doi: 10.1002/bip.22278. Biopolymers. 2014. PMID: 23653336
-
Micellar systems as solvents in peptide and protein structure determination.Methods Enzymol. 2001;339:271-85. doi: 10.1016/s0076-6879(01)39318-7. Methods Enzymol. 2001. PMID: 11462816 Review. No abstract available.
-
An evaluation of peptide-bond isosteres.Chembiochem. 2011 Aug 16;12(12):1801-7. doi: 10.1002/cbic.201100272. Epub 2011 Jul 12. Chembiochem. 2011. PMID: 21751326 Free PMC article. Review.
Cited by
-
Replacing a single atom accelerates the folding of a protein and increases its thermostability.Org Biomol Chem. 2016 Jul 12;14(28):6780-5. doi: 10.1039/c6ob00980h. Org Biomol Chem. 2016. PMID: 27336677 Free PMC article.
-
Synthesis of 5-fluoro- and 5-hydroxymethanoprolines via lithiation of N-BOC-methanopyrrolidines. Constrained Cγ-exo and Cγ-endo Flp and Hyp conformer mimics.J Org Chem. 2012 Jun 15;77(12):5331-44. doi: 10.1021/jo300700a. Epub 2012 May 25. J Org Chem. 2012. PMID: 22607128 Free PMC article.
-
n→π* Interactions Are Competitive with Hydrogen Bonds.Org Lett. 2016 Aug 5;18(15):3614-7. doi: 10.1021/acs.orglett.6b01655. Epub 2016 Jul 13. Org Lett. 2016. PMID: 27409515 Free PMC article.
-
Non-covalent interactions: Fold globally, bond locally.Nat Chem Biol. 2010 Aug;6(8):567-8. doi: 10.1038/nchembio.413. Nat Chem Biol. 2010. PMID: 20644541 No abstract available.
-
An n→π* interaction reduces the electrophilicity of the acceptor carbonyl group.Chem Commun (Camb). 2013 Sep 25;49(74):8166-8. doi: 10.1039/c3cc44573a. Chem Commun (Camb). 2013. PMID: 23928794 Free PMC article.
References
-
- An, S.S.A., Lester, C.C., Peng, J.-L., Li, Y.-J., Rothwarf, D.M., Welker, E., Thannhauser, T.W., Zhang, L.S., Tam, J.P., and Scheraga, H.A. 1999. Retention of the cis proline conformation in tripeptide fragments of bovine pancreatic ribonuclease A containing a non-natural proline analogue, 5,5-dimethylproline. J. Am. Chem. Soc. 121 11558–11566.
-
- Antonyraj, K.J., Karunakaran, T., and Raj, P.A. 1998. Bactericidal activity and poly-L-proline II conformation of the tandem repeat sequence of human salivary mucin glycoprotein (MG2). Arch. Biochem. Biophys. 356 197–206. - PubMed
-
- Baldwin, J.E. 1976. Approach vector analysis: A stereochemical approach to reactivity. Chem. Commun. 738–741.
-
- Bella, J., Eaton, M., Brodsky, B., and Berman, H.M. 1994. Crystal and molecular structure of a collagen-like peptide at 1.9 Å resolution. Science 266 75–81. - PubMed
-
- Benzi, C., Improta, R., Scalmani, G., and Barone, V. 2002. Quantum mechanical study of the conformational behavior of proline and 4R-hydroxyproline dipeptide analogues in vacuum and in aqueous solution. J. Comput. Chem. 23 341–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

