Structural perturbation and enhancement of the chaperone-like activity of alpha-crystallin by arginine hydrochloride
- PMID: 12761397
- PMCID: PMC2323889
- DOI: 10.1110/ps.0302003
Structural perturbation and enhancement of the chaperone-like activity of alpha-crystallin by arginine hydrochloride
Abstract
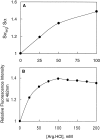
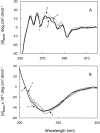
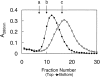
Structural perturbation of alpha-crystallin is shown to enhance its molecular chaperone-like activity in preventing aggregation of target proteins. We demonstrate that arginine, a biologically compatible molecule that is known to bind to the peptide backbone and negatively charged side-chains, increases the chaperone-like activity of calf eye lens alpha-crystallin as well as recombinant human alphaA- and alphaB-crystallins. Arginine-induced increase in the chaperone activity is more pronounced for alphaB-crystallin than for alphaA-crystallin. Other guanidinium compounds such as aminoguanidine hydrochloride and guanidine hydrochloride also show a similar effect, but to different extents. A point mutation, R120G, in alphaB-crystallin that is associated with desmin-related myopathy, results in a significant loss of chaperone-like activity. Arginine restores the activity of mutant protein to a considerable extent. We have investigated the effect of arginine on the structural changes of alpha-crystallin by circular dichroism, fluorescence, and glycerol gradient sedimentation. Far-UV CD spectra show no significant changes in secondary structure, whereas near-UV CD spectra show subtle changes in the presence of arginine. Glycerol gradient sedimentation shows a significant decrease in the size of alpha-crystallin oligomer in the presence of arginine. Increased exposure of hydrophobic surfaces of alpha-crystallin, as monitored by pyrene-solubilization and ANS-fluorescence, is observed in the presence of arginine. These results show that arginine brings about subtle changes in the tertiary structure and significant changes in the quaternary structure of alpha-crystallin and enhances its chaperone-like activity significantly. This study should prove useful in designing strategies to improve chaperone function for therapeutic applications.
Figures

Similar articles
-
Arginine hydrochloride enhances the dynamics of subunit assembly and the chaperone-like activity of alpha-crystallin.Mol Vis. 2005 Apr 1;11:249-55. Mol Vis. 2005. PMID: 15827547
-
Structural and functional consequences of the mutation of a conserved arginine residue in alphaA and alphaB crystallins.J Biol Chem. 1999 Aug 20;274(34):24137-41. doi: 10.1074/jbc.274.34.24137. J Biol Chem. 1999. PMID: 10446186
-
The IXI/V motif in the C-terminal extension of alpha-crystallins: alternative interactions and oligomeric assemblies.Mol Vis. 2004 Sep 8;10:655-62. Mol Vis. 2004. PMID: 15448619
-
Structural perturbation of alpha-crystallin and its chaperone-like activity.Int J Biol Macromol. 1998 May-Jun;22(3-4):271-81. doi: 10.1016/s0141-8130(98)00025-7. Int J Biol Macromol. 1998. PMID: 9650082 Review.
-
Alpha-crystallin as a molecular chaperone.Prog Retin Eye Res. 1999 Jul;18(4):463-509. doi: 10.1016/s1350-9462(98)00030-5. Prog Retin Eye Res. 1999. PMID: 10217480 Review.
Cited by
-
HSPB5 suppresses renal inflammation and protects lupus-prone NZB/W F1 mice from severe renal damage.Arthritis Res Ther. 2022 Dec 12;24(1):267. doi: 10.1186/s13075-022-02958-9. Arthritis Res Ther. 2022. PMID: 36510250 Free PMC article.
-
Functional Rescue of Cataract-Causing αA-G98R-Crystallin by Targeted Compensatory Suppressor Mutations in Human αA-Crystallin.Biochemistry. 2019 Oct 8;58(40):4148-4158. doi: 10.1021/acs.biochem.9b00374. Epub 2019 Sep 20. Biochemistry. 2019. PMID: 31523965 Free PMC article.
-
Arginine and the Hofmeister Series: the role of ion-ion interactions in protein aggregation suppression.J Phys Chem B. 2011 Jun 9;115(22):7447-58. doi: 10.1021/jp111920y. Epub 2011 May 13. J Phys Chem B. 2011. PMID: 21568311 Free PMC article.
-
Effect of Betaine and Arginine on Interaction of αB-Crystallin with Glycogen Phosphorylase b.Int J Mol Sci. 2022 Mar 30;23(7):3816. doi: 10.3390/ijms23073816. Int J Mol Sci. 2022. PMID: 35409175 Free PMC article.
-
Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-crystallin.Biochemistry. 2009 Jun 16;48(23):5066-73. doi: 10.1021/bi900085v. Biochemistry. 2009. PMID: 19388699 Free PMC article.
References
-
- Aoyama, A., Steiger, R.H., Frohli, E., Schafer, R., Von Deimling, A., Wiestler, O.D.M., and Klemenz, R. 1993. Expression of α B-crystallin in human brain tumors. Int. J. Cancer 55 760–764. - PubMed
-
- Arakawa, T. and Timasheff, S.N. 1984. Protein stabilization and destabilization by guanidinium salts. Biochemistry 23 5924–5929. - PubMed
-
- Bhat, S.P. and Nagineni, C.N. 1989. α B subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem. Biophys. Res. Commun. 158 319–325. - PubMed
-
- Borkman, R.F., Knight, G., and Obi, B. 1996. The molecular chaperone αcrystallin inhibits UV-induced protein aggregation. Exp. Eye Res. 62 141–148. - PubMed
-
- Bova, M.P., Yaron, O., Huang, Q.L., Ding, L.L., Haley, D.A., Stewart, P.L., and Horwitz, J. 1999. Mutation R120G in αB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl. Acad. Sci. 96 6137–6142. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

