Nucleotide coronary vasodilation in guinea pig hearts
- PMID: 12763753
- PMCID: PMC8620194
- DOI: 10.1152/ajpheart.00981.2002
Nucleotide coronary vasodilation in guinea pig hearts
Abstract
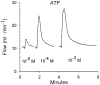
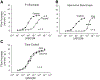
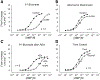
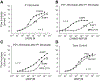
The role of P1 receptors and P2Y1 receptors in coronary vasodilator responses to adenine nucleotides was examined in the isolated guinea pig heart. Bolus arterial injections of nucleotides were made in hearts perfused at constant pressure. Peak increase in flow was measured before and after addition of purinoceptor antagonists. Both the P1 receptor antagonist 8-(p-sulfophenyl)theophylline and adenosine deaminase inhibited adenosine vasodilation. AMP-induced vasodilation was inhibited by P1 receptor blockade but not by adenosine deaminase or by the selective P2Y1 antagonist N6-methyl-2'-deoxyadenosine 3',5'-bisphosphate (MRS 2179). ADP-induced vasodilation was moderately inhibited by P1 receptor blockade and greatly inhibited by combined P1 and P2Y1 blockade. ATP-induced vasodilation was antagonized by P1 blockade but not by adenosine deaminase. Addition of P2Y1 blockade to P1 blockade shifted the ATP dose-response curve further rightward. It is concluded that in this preparation ATP-induced vasodilation results primarily from AMP stimulation of P1 receptors, with a smaller component from ATP or ADP acting on P2Y1 receptors. ADP-induced vasodilation is largely due to P2Y1 receptors, with a smaller contribution by AMP or adenosine acting via P1 receptors. AMP responses are mediated solely by P1 receptors. Adenosine contributes very little to vasodilation resulting from bolus intracoronary injections of ATP, ADP, or AMP.
Figures

References
-
- Belardinelli L, Shryock J, West A, Clemo HF, DiMarco JP, and Berne RM. Effects of adenosine and adenine nucleotides on the atrioventricular node of isolated guinea pig hearts. Circulation 70: 1083–1091, 1984. - PubMed
-
- Bergfeld GR and Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992. - PubMed
-
- Borst MM and Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res 68: 797–806, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

