High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis
- PMID: 12763935
- PMCID: PMC2963562
- DOI: 10.1182/blood-2002-12-3908
High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis
Abstract
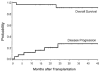
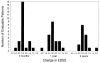
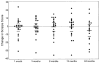
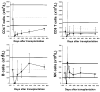
There were 26 patients enrolled in a pilot study of high-dose immunosuppressive therapy (HDIT) for severe multiple sclerosis (MS). Median baseline expanded disability status scale (EDSS) was 7.0 (range, 5.0-8.0). HDIT consisted of total body irradiation, cyclophosphamide, and antithymocyte globulin (ATG) and was followed by transplantation of autologous, granulocyte colony-stimulating factor (G-CSF)-mobilized CD34-selected stem cells. Regimen-related toxicities were mild. Because of bladder dysfunction, there were 8 infectious events of the lower urinary tract. One patient died from Epstein-Barr virus (EBV)-related posttransplantation lymphoproliferative disorder (PTLD) associated with a change from horse-derived to rabbit-derived ATG in the HDIT regimen. An engraftment syndrome characterized by noninfectious fever with or without rash developed in 13 of the first 18 patients and was associated in some cases with transient worsening of neurologic symptoms. There were 2 significant adverse neurologic events that occurred, including a flare of MS during mobilization and an episode of irreversible neurologic deterioration after HDIT associated with fever. With a median follow-up of 24 (range, 3-36) months, the Kaplan-Meier estimate of progression (>/= 1.0 point EDSS) at 3 years was 27%. Of 12 patients who had oligoclonal bands in the cerebrospinal fluid at baseline, 9 had persistence after HDIT. After HDIT, 4 patients developed new enhancing lesions on magnetic resonance imaging of the brain. The estimate of survival at 3 years was 91%. Important clinical issues in the use of HDIT and stem cell transplantation for MS were identified; however, modifications of the initial approaches appear to reduce treatment risks. This was a heterogeneous high-risk group, and a phase 3 study is planned to fully assess efficacy.
Figures
References
-
- Kurtzke JF, Beebe GW, Nagler B, Kurland LT, Auth TL. Studies on the natural history of multiple sclerosis-8: early prognostic features of the later course of the illness. J Chronic Dis. 1977;30:819–830. - PubMed
-
- Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain. 1980;103:281–300. - PubMed
-
- Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain. 1993;116:117–134. - PubMed
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: implications for counselling and therapy [review] Curr Opin Neurol. 2002;15:257–266. - PubMed
-
- Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–1438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

