ABC of interventional cardiology: percutaneous coronary intervention. II: the procedure
- PMID: 12763994
- PMCID: PMC514052
- DOI: 10.1136/bmj.326.7399.1137
ABC of interventional cardiology: percutaneous coronary intervention. II: the procedure
Figures












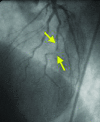


Similar articles
-
We can build it, but will they come?Catheter Cardiovasc Interv. 2011 May 1;77(6):818-9. doi: 10.1002/ccd.23131. Catheter Cardiovasc Interv. 2011. PMID: 21520383 No abstract available.
-
If I've heard it once, I've heard it (not yet) a hundred times.Catheter Cardiovasc Interv. 2011 Mar 1;77(4):502. doi: 10.1002/ccd.22989. Catheter Cardiovasc Interv. 2011. PMID: 21351224 No abstract available.
-
Optimizing the impact of primary percutaneous coronary intervention in acute myocardial infarction.Catheter Cardiovasc Interv. 2011 Feb 1;77(2):201. doi: 10.1002/ccd.22952. Catheter Cardiovasc Interv. 2011. PMID: 21290554 No abstract available.
-
[Percutaneous coronary interventions for acute coronary syndrome and left coronary arterial trunk lesion: state-of-the-art].Vestn Rentgenol Radiol. 2011 Jul-Aug;(3):64-8. Vestn Rentgenol Radiol. 2011. PMID: 22288138 Review. Russian. No abstract available.
-
ABC of interventional cardiology: percutaneous coronary intervention. I: history and development.BMJ. 2003 May 17;326(7398):1080-2. doi: 10.1136/bmj.326.7398.1080. BMJ. 2003. PMID: 12750213 Free PMC article. Review. No abstract available.
Cited by
-
Impact of renin angiotensin system inhibitor on 3-year clinical outcomes in acute myocardial infarction patients with preserved left ventricular systolic function: a prospective cohort study from Korea Acute Myocardial Infarction Registry (KAMIR).BMC Cardiovasc Disord. 2021 May 21;21(1):251. doi: 10.1186/s12872-021-02070-x. BMC Cardiovasc Disord. 2021. PMID: 34020593 Free PMC article.
-
Antegrade balloon occlusion of inferior vena cava during thrombectomy for renal cell carcinoma.Can Urol Assoc J. 2010 Aug;4(4):E105-8. doi: 10.5489/cuaj.892. Can Urol Assoc J. 2010. PMID: 20694087 Free PMC article.
-
ST-elevation versus non-ST-elevation myocardial infarction after combined use of statin with renin-angiotensin system inhibitor: Data from the Korea Acute Myocardial Infarction Registry.Cardiol J. 2022;29(4):647-659. doi: 10.5603/CJ.a2021.0007. Epub 2021 Feb 26. Cardiol J. 2022. PMID: 33634844 Free PMC article.
-
Incremental predictive value of the combined use of the neutrophil-to-lymphocyte ratio and systolic blood pressure difference after successful drug-eluting stent implantation.Cardiol J. 2023;30(1):91-104. doi: 10.5603/CJ.a2021.0004. Epub 2021 Jan 13. Cardiol J. 2023. PMID: 33438177 Free PMC article.
-
Xiongshao for restenosis after percutaneous coronary intervention in patients with coronary heart disease.Cochrane Database Syst Rev. 2013 May 31;2013(5):CD009581. doi: 10.1002/14651858.CD009581.pub2. Cochrane Database Syst Rev. 2013. PMID: 23728695 Free PMC article.
References
-
- Smith SC Jr, Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, et al. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines)—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty). J Am Coll Cardiol 2001;37: 2215-39 - PubMed
-
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346: 1773-80 - PubMed
-
- Almond DG. Coronary stenting I: intracoronary stents—form, function future. In: Grech ED, Ramsdale DR, eds. Practical interventional cardiology. 2nd ed. London: Martin Dunitz, 2002: 63-76
-
- Waksman R. Management of restenosis through radiation therapy. In: Grech ED, Ramsdale DR, eds. Practical interventional cardiology. 2nd ed. London: Martin Dunitz, 2002: 295-305
-
- Kimmel SE, Berlin JA, Laskey WK. The relationship between coronary angioplasty procedure volume and major complications. JAMA 1995;274: 1137-42 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
