Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade
- PMID: 12764090
- PMCID: PMC6741112
- DOI: 10.1523/JNEUROSCI.23-10-04034.2003
Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade
Abstract
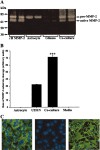
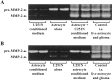
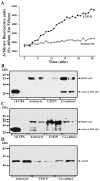
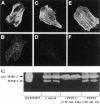
The presence of reactive astrocytes around glioma cells in the CNS suggests the possibility that these two cell types could be interacting. We addressed whether glioma cells use the astrocyte environment to modulate matrix metalloproteinase-2 (MMP-2), a proteolytic enzyme implicated in the invasiveness of glioma cells. We found that astrocytes in culture produce significant amounts of the pro-form of MMP-2 but undetectable levels of active MMP-2. However, after coculture with the U251N glioma line, astrocyte pro-MMP-2 was converted to the active form. The mechanism of pro-MMP-2 activation in glioma-astrocyte coculture was investigated and was found to involve the urokinase-type plasminogen activator (uPA)-plasmin cascade whereby uPA bound to uPA receptor (uPAR), leading to the conversion of plasminogen to plasmin. The latter cleaved pro-MMP-2 to generate its active form. Furthermore, key components (i.e., uPAR, uPA, and pro-MMP-2) were contributed principally by astrocytes, whereas the U251N glioma cells provided plasminogen. In correspondence with this biochemical cascade, the transmigration of U251N cells through Boyden invasion chambers coated with an extracellular matrix barrier was increased significantly in the presence of astrocytes, and this was inhibited by agents that disrupted the uPA-plasmin cascade. Finally, using resected human glioblastoma specimens, we found that tumor cells, but not astrocytes, expressed plasminogen in situ. We conclude that glioma cells exploit their astrocyte environment to activate MMP-2 and that this leads to the increased invasiveness of glioma cells.
Figures




Similar articles
-
Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis.Clin Cancer Res. 2007 Jun 1;13(11):3115-24. doi: 10.1158/1078-0432.CCR-06-2088. Clin Cancer Res. 2007. PMID: 17545513
-
Kinetics of reciprocal pro-urokinase/plasminogen activation--stimulation by a template formed by the urokinase receptor bound to poly(D-lysine).Eur J Biochem. 1997 Apr 15;245(2):316-23. doi: 10.1111/j.1432-1033.1997.00316.x. Eur J Biochem. 1997. PMID: 9151959
-
Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants.EMBO J. 1997 May 1;16(9):2319-32. doi: 10.1093/emboj/16.9.2319. EMBO J. 1997. PMID: 9171346 Free PMC article.
-
[Mechanism of tumor cell-induced extracellular matrix degradation--inhibition of cell-surface proteolytic activity might have a therapeutic effect on tumor cell invasion and metastasis].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):623-32. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808830 Review. Japanese.
-
[The clinical prospects for the study of the plasminogen activation system in breast cancer].Vestn Ross Akad Med Nauk. 1999;(8):58-61. Vestn Ross Akad Med Nauk. 1999. PMID: 10487126 Review. Russian.
Cited by
-
Bcl-w Enhances Mesenchymal Changes and Invasiveness of Glioblastoma Cells by Inducing Nuclear Accumulation of β-Catenin.PLoS One. 2013 Jun 27;8(6):e68030. doi: 10.1371/journal.pone.0068030. Print 2013. PLoS One. 2013. PMID: 23826359 Free PMC article.
-
Glioblastoma Standard of Care: Effects on Tumor Evolution and Reverse Translation in Preclinical Models.Cancers (Basel). 2024 Jul 24;16(15):2638. doi: 10.3390/cancers16152638. Cancers (Basel). 2024. PMID: 39123366 Free PMC article. Review.
-
Rapid Reprogramming of Primary Human Astrocytes into Potent Tumor-Initiating Cells with Defined Genetic Factors.Cancer Res. 2016 Sep 1;76(17):5143-50. doi: 10.1158/0008-5472.CAN-16-0171. Epub 2016 Jun 30. Cancer Res. 2016. PMID: 27364552 Free PMC article.
-
The perivascular niche microenvironment in brain tumor progression.Cell Cycle. 2010 Aug 1;9(15):3012-21. doi: 10.4161/cc.9.15.12710. Cell Cycle. 2010. PMID: 20714216 Free PMC article. Review.
-
Protease activity of 80 kDa protein secreted from the apicomplexan parasite Toxoplasma gondii.Korean J Parasitol. 2003 Sep;41(3):165-9. doi: 10.3347/kjp.2003.41.3.165. Korean J Parasitol. 2003. PMID: 12972730 Free PMC article.
References
-
- Apodaca G, Rutka JT, Bouhana K, Berens ME, Giblin JR, Rosenblum ML, McKerrow JH, Banda MJ ( 1990) Expression of metalloproteinases and metalloproteinase inhibitors by fetal astrocytes and glioma cells. Cancer Res 50: 2322–2329. - PubMed
-
- Beliveau R, Delbecchi L, Beaulieu E, Mousseau N, Kachra Z, Berthelet F, Moumdjian R, Del Maestro R ( 1999) Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Ann NY Acad Sci 886: 236–239. - PubMed
-
- Bello L, Lucini V, Carrabba G, Giussani C, Machluf M, Pluderi M, Nikas D, Zhang J, Tomei G, Villani RM, Carroll RS, Bikfalvi A, Black PM ( 2001) Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res 61: 8730–8736. - PubMed
-
- Benbow U, Schoenermark MP, Mitchell TI, Rutter JL, Shimokawa K, Nagase H, Brinckerhoff CE ( 1999) A novel host/tumor cell interaction activates matrix metalloproteinase-1 and mediates invasion through type I collagen. J Biol Chem 274: 25371–25378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
