The alpha-latrotoxin mutant LTXN4C enhances spontaneous and evoked transmitter release in CA3 pyramidal neurons
- PMID: 12764091
- PMCID: PMC6741093
- DOI: 10.1523/JNEUROSCI.23-10-04044.2003
The alpha-latrotoxin mutant LTXN4C enhances spontaneous and evoked transmitter release in CA3 pyramidal neurons
Abstract
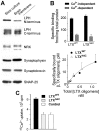
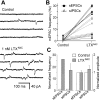
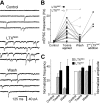
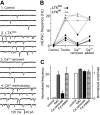
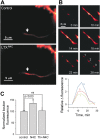
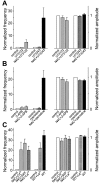
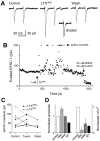
Alpha-latrotoxin (LTX) stimulates vesicular exocytosis by at least two mechanisms that include (1) receptor binding-stimulation and (2) membrane pore formation. Here, we use the toxin mutant LTX(N4C) to selectively study the receptor-mediated actions of LTX. LTX(N4C) binds to both LTX receptors (latrophilin and neurexin) and greatly enhances the frequency of spontaneous and miniature EPSCs recorded from CA3 pyramidal neurons in hippocampal slice cultures. The effect of LTX(N4C) is reversible and is not attenuated by La3+ that is known to block LTX pores. On the other hand, LTX(N4C) action, which requires extracellular Ca2+, is inhibited by thapsigargin, a drug depleting intracellular Ca2+ stores, by 2-aminoethoxydiphenyl borate, a blocker of inositol(1,4,5)-trisphosphate-induced Ca2+ release, and by U73122, a phospholipase C inhibitor. Furthermore, measurements using a fluorescent Ca2+ indicator directly demonstrate that LTX(N4C) increases presynaptic, but not dendritic, free Ca2+ concentration; this Ca2+ rise is blocked by thapsigargin, suggesting, together with electrophysiological data, that the receptor-mediated action of LTX(N4C) involves mobilization of Ca2+ from intracellular stores. Finally, in contrast to wild-type LTX, which inhibits evoked synaptic transmission probably attributable to pore formation, LTX(N4C) actually potentiates synaptic currents elicited by electrical stimulation of afferent fibers. We suggest that the mutant LTX(N4C), lacking the ionophore-like activity of wild-type LTX, activates a presynaptic receptor and stimulates Ca2+ release from intracellular stores, leading to the enhancement of synaptic vesicle exocytosis.
Figures

References
-
- Ashton AC, Rahman MA, Volynski KE, Manser C, Orlova EV, Matsushita H, Davletov BA, van Heel M, Grishin EV, Ushkaryov YA ( 2000) Tetramerisation of α-latrotoxin by divalent cations is responsible for toxin-induced non-vesicular release and contributes to the Ca2+-dependent vesicular exocytosis from synaptosomes. Biochimie 82: 453–468. - PubMed
-
- Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA ( 2001) α-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem 276: 44695–44703. - PubMed
-
- Capogna M, Gähwiler BH, Thompson SM ( 1996a) Presynaptic inhibition of calcium-dependent and -independent release elicited with ionomycin, gadolinium, and α-latrotoxin in the hippocampus. J Neurophysiol 75: 2017–2028. - PubMed
-
- Capogna M, Gähwiler BH, Thompson SM ( 1996b) Calcium-independent actions of α-latrotoxin on spontaneous and evoked synaptic transmission in the hippocampus. J Neurophysiol 76: 3149–3158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
