Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem
- PMID: 12764101
- PMCID: PMC6741087
- DOI: 10.1523/JNEUROSCI.23-10-04134.2003
Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem
Abstract
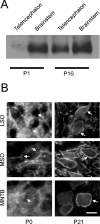
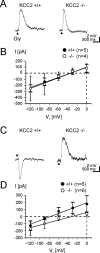
Glycine and GABA, the dominant inhibitory neurotransmitters in the CNS, assume a depolarizing role in early development, leading to increased cytoplasmic Ca2+ levels and action potentials. The effect is thought to be of some significance for maturation. The depolarization is caused by Cl- efflux, and chloride transporters contribute to the phenomenon by raising the intracellular Cl- concentration ([Cl-]i) above equilibrium, thereby generating an outward-directed electrochemical gradient for Cl-. In mature neurons, the [Cl-]i is reduced below equilibrium, thus rendering glycine activity hyperpolarizing. Here, we investigated the temporal expression of the K-Cl cotransporter KCC2 and the Na-K-Cl cotransporter NKCC1 in the lateral superior olive (LSO) of rats and mice. The two cation cotransporters normally extrude and accumulate Cl-, respectively. As evidenced by several methods, KCC2 mRNA was present in LSO neurons during both the depolarizing and hyperpolarizing periods. Western blots confirmed a constant level of KCC2 in the brainstem, and immunohistochemistry showed that the protein is diffusely distributed within neonatal LSO neurons, becoming integrated into the plasma membrane only with increasing age. The glycine reversal potential in KCC2 knock-out mice differed significantly from that determined in wild-type controls at postnatal day 12 (P12) but not at P3, demonstrating that KCC2 is not active in neonates, despite its early presence. NKCC1 mRNA was not detected during the depolarizing phase in the LSO, implying that this transporter does not contribute to the high [Cl-]i. Our results reveal major differences in the development of [Cl-]i regulation mechanisms seen in brainstem versus forebrain regions.
Figures


Similar articles
-
Hypothyroidism impairs chloride homeostasis and onset of inhibitory neurotransmission in developing auditory brainstem and hippocampal neurons.Eur J Neurosci. 2008 Dec;28(12):2371-80. doi: 10.1111/j.1460-9568.2008.06528.x. Eur J Neurosci. 2008. PMID: 19087168
-
Oligomerization of KCC2 correlates with development of inhibitory neurotransmission.J Neurosci. 2006 Oct 11;26(41):10407-19. doi: 10.1523/JNEUROSCI.3257-06.2006. J Neurosci. 2006. PMID: 17035525 Free PMC article.
-
Glycinergic and GABAergic calcium responses in the developing lateral superior olive.Eur J Neurosci. 2002 Apr;15(7):1093-104. doi: 10.1046/j.1460-9568.2002.01946.x. Eur J Neurosci. 2002. PMID: 11982621 Free PMC article.
-
The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures.Neurosurg Focus. 2008 Sep;25(3):E22. doi: 10.3171/FOC/2008/25/9/E22. Neurosurg Focus. 2008. PMID: 18759624 Review.
-
Roles of the cation-chloride cotransporters in neurological disease.Nat Clin Pract Neurol. 2008 Sep;4(9):490-503. doi: 10.1038/ncpneuro0883. Nat Clin Pract Neurol. 2008. PMID: 18769373 Review.
Cited by
-
Chloride Homeostasis in Developing Motoneurons.Adv Neurobiol. 2022;28:45-61. doi: 10.1007/978-3-031-07167-6_2. Adv Neurobiol. 2022. PMID: 36066820
-
Time-dependent gene expression analysis of the developing superior olivary complex.J Biol Chem. 2013 Sep 6;288(36):25865-25879. doi: 10.1074/jbc.M113.490508. Epub 2013 Jul 26. J Biol Chem. 2013. PMID: 23893414 Free PMC article.
-
Novel determinants of the neuronal Cl(-) concentration.J Physiol. 2014 Oct 1;592(19):4099-114. doi: 10.1113/jphysiol.2014.275529. Epub 2014 Aug 8. J Physiol. 2014. PMID: 25107928 Free PMC article. Review.
-
KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses.Eur J Neurosci. 2005 May;21(9):2593-9. doi: 10.1111/j.1460-9568.2005.04084.x. Eur J Neurosci. 2005. PMID: 15932617 Free PMC article.
-
Acoustic trauma slows AMPA receptor-mediated EPSCs in the auditory brainstem, reducing GluA4 subunit expression as a mechanism to rescue binaural function.J Physiol. 2016 Jul 1;594(13):3683-703. doi: 10.1113/JP271929. Epub 2016 Jun 9. J Physiol. 2016. PMID: 27104476 Free PMC article.
References
-
- Alvarez-Leefmans FJ ( 2001) Intracellular chloride regulation. In: Cell physiology sourcebook: a molecular approach (Sperelakis N, ed), pp 301–318. San Diego: Academic.
-
- Barker JL, Behar T, Li YX, Liu QY, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen XL, Xian H ( 1998) GABAergic cells and signals in CNS development. Perspect Dev Neurobiol 5: 305–322. - PubMed
-
- Becker M, Nothwang HG, Friauf E ( 2003) Differential expression pattern of chloride transporters NCC, NKCC2, KCC1, KCC3, KCC4, and AE3 in the developing rat auditory brainstem. Cell Tiss Res, in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
