Synapse number and synaptic efficacy are regulated by presynaptic cAMP and protein kinase A
- PMID: 12764102
- PMCID: PMC6741068
- DOI: 10.1523/JNEUROSCI.23-10-04146.2003
Synapse number and synaptic efficacy are regulated by presynaptic cAMP and protein kinase A
Abstract
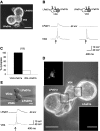
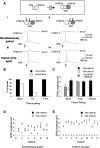
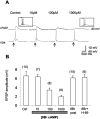
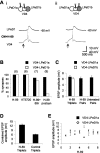
The mechanisms by which neurons regulate the number and strength of synapses during development and synaptic plasticity have not yet been defined fully. This lack of fundamental knowledge in the fields of neurodevelopment and synaptic plasticity can be attributed, in part, to compensatory mechanisms by which neurons accommodate for the loss of function in their synaptic partners. This is generally achieved either by scaling up neuronal transmitter release capabilities or by enhancing the postsynaptic responsiveness. Here, we demonstrate that regulation of synaptic strength and number between identified Lymnaea neurons visceral dorsal 4 (VD4, the presynaptic cell) and left pedal dorsal 1 (LPeD1, the postsynaptic cell) requires presynaptic activation of a cAMP-PKA-dependent signal. Experimental activation of the cAMP-PKA pathway resulted in reduced synaptic efficacy, whereas inhibition of the cAMP-PKA cascade permitted hyperinnervation and an overall enhancement of synaptic strength. Because synaptic transmission between VD4 and LPeD1 does not require a cAMP-PKA pathway, our data show that these messengers may play a novel role in regulating the synaptic efficacy during early synaptogenesis and plasticity.
Figures

Similar articles
-
Serotonin modulates transmitter release at central Lymnaea synapses through a G-protein-coupled and cAMP-mediated pathway.Eur J Neurosci. 2008 Apr;27(8):2033-42. doi: 10.1111/j.1460-9568.2008.06180.x. Eur J Neurosci. 2008. PMID: 18412624
-
Trophic factor-induced excitatory synaptogenesis involves postsynaptic modulation of nicotinic acetylcholine receptors.J Neurosci. 2002 Jan 15;22(2):505-14. doi: 10.1523/JNEUROSCI.22-02-00505.2002. J Neurosci. 2002. PMID: 11784796 Free PMC article.
-
A novel form of presynaptic CaMKII-dependent short-term potentiation between Lymnaea neurons.Eur J Neurosci. 2011 Aug;34(4):569-77. doi: 10.1111/j.1460-9568.2011.07784.x. Epub 2011 Jul 12. Eur J Neurosci. 2011. PMID: 21749498
-
Signaling from cAMP/PKA to MAPK and synaptic plasticity.Mol Neurobiol. 2003 Feb;27(1):99-106. doi: 10.1385/MN:27:1:99. Mol Neurobiol. 2003. PMID: 12668903 Review.
-
Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases.Prog Neurobiol. 2003 Dec;71(6):401-37. doi: 10.1016/j.pneurobio.2003.12.003. Prog Neurobiol. 2003. PMID: 15013227 Review.
Cited by
-
cAMP-Inhibits Cytoplasmic Phospholipase A₂ and Protects Neurons against Amyloid-β-Induced Synapse Damage.Biology (Basel). 2015 Sep 16;4(3):591-606. doi: 10.3390/biology4030591. Biology (Basel). 2015. PMID: 26389963 Free PMC article.
-
Involvement of the cAMP-dependent pathway in the reduction of epileptiform bursting caused by somatostatin in the mouse hippocampus.Naunyn Schmiedebergs Arch Pharmacol. 2008 Dec;378(6):563-77. doi: 10.1007/s00210-008-0338-z. Epub 2008 Jul 30. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18665350
-
Synaptogenesis in the CNS: an odyssey from wiring together to firing together.J Physiol. 2003 Oct 1;552(Pt 1):1-11. doi: 10.1113/jphysiol.2003.045062. Epub 2003 Aug 1. J Physiol. 2003. PMID: 12897180 Free PMC article. Review.
-
Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring.Immunity. 2021 Nov 9;54(11):2611-2631.e8. doi: 10.1016/j.immuni.2021.10.006. Immunity. 2021. PMID: 34758338 Free PMC article.
-
Shared and unique phosphoproteomics responses in skeletal muscle from exercise models and in hyperammonemic myotubes.iScience. 2022 Oct 10;25(11):105325. doi: 10.1016/j.isci.2022.105325. eCollection 2022 Nov 18. iScience. 2022. PMID: 36345342 Free PMC article.
References
-
- Ahmari SE, Smith SJ ( 2002) Knowing a nascent synapse when you see it. Neuron 34: 333–336. - PubMed
-
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER ( 1999) A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221–237. - PubMed
-
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD ( 2000) Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27: 107–119. - PubMed
-
- Colman H, Nabekura J, Lichtman JW ( 1997) Alterations in synaptic strength preceding axon withdrawal. Science 275: 356–361. - PubMed
-
- Davis GW, Bezprozvanny I ( 2001) Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol 63: 847–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
