Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury
- PMID: 12764110
- PMCID: PMC6741116
- DOI: 10.1523/JNEUROSCI.23-10-04219.2003
Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury
Abstract
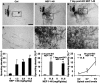
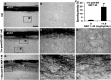
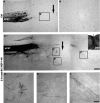
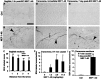
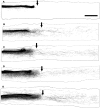
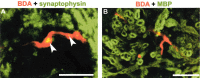
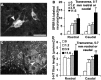
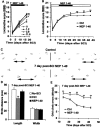
Traumatized axons possess an extremely limited ability to regenerate within the adult mammalian CNS. The myelin-derived axon outgrowth inhibitors Nogo, oligodendrocyte-myelin glycoprotein, and myelin-associated glycoprotein, all bind to an axonal Nogo-66 receptor (NgR) and at least partially account for this lack of CNS repair. Although the intrathecal application of an NgR competitive antagonist at the time of spinal cord hemisection induces significant regeneration of corticospinal axons, such immediate local therapy may not be as clinically feasible for cases of spinal cord injury. Here, we consider whether this approach can be adapted to systemic therapy in a postinjury therapeutic time window. Subcutaneous treatment with the NgR antagonist peptide NEP1-40 (Nogo extracellular peptide, residues 1-40) results in extensive growth of corticospinal axons, sprouting of serotonergic fibers, upregulation of axonal growth protein SPRR1A (small proline-rich repeat protein 1A), and synapse re-formation. Locomotor recovery after thoracic spinal cord injury is enhanced. Furthermore, delaying the initiation of systemic NEP1-40 administration for up to 1 week after cord lesions does not limit the degree of axon sprouting and functional recovery. This indicates that the regenerative capacity of transected corticospinal tract axons persists for weeks after injury. Systemic Nogo-66 receptor antagonists have therapeutic potential for subacute CNS axonal injuries such as spinal cord trauma.
Figures
Similar articles
-
Nogo-66 receptor antagonist peptide promotes axonal regeneration.Nature. 2002 May 30;417(6888):547-51. doi: 10.1038/417547a. Nature. 2002. PMID: 12037567
-
Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury.Neuron. 2004 Oct 28;44(3):439-51. doi: 10.1016/j.neuron.2004.10.015. Neuron. 2004. PMID: 15504325
-
Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat.Neurorehabil Neural Repair. 2008 May-Jun;22(3):262-78. doi: 10.1177/1545968307308550. Epub 2007 Nov 30. Neurorehabil Neural Repair. 2008. PMID: 18056009 Free PMC article.
-
The Nogo receptor, its ligands and axonal regeneration in the spinal cord; a review.J Neurocytol. 2002 Feb;31(2):93-120. doi: 10.1023/a:1023941421781. J Neurocytol. 2002. PMID: 12815233 Review.
-
Why do Nogo/Nogo-66 receptor gene knockouts result in inferior regeneration compared to treatment with neutralizing agents?J Neurochem. 2005 Aug;94(4):865-74. doi: 10.1111/j.1471-4159.2005.03238.x. J Neurochem. 2005. PMID: 16092935 Review.
Cited by
-
Gene expression changes in spinal motoneurons of the SOD1(G93A) transgenic model for ALS after treatment with G-CSF.Front Cell Neurosci. 2015 Jan 20;8:464. doi: 10.3389/fncel.2014.00464. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25653590 Free PMC article.
-
Derivation of multivariate syndromic outcome metrics for consistent testing across multiple models of cervical spinal cord injury in rats.PLoS One. 2013;8(3):e59712. doi: 10.1371/journal.pone.0059712. Epub 2013 Mar 27. PLoS One. 2013. PMID: 23544088 Free PMC article.
-
Expression Pattern of 5-HT (Serotonin) Receptors during Normal Development of the Human Spinal Cord and Ganglia and in Fetus with Cervical Spina Bifida.Int J Mol Sci. 2021 Jul 7;22(14):7320. doi: 10.3390/ijms22147320. Int J Mol Sci. 2021. PMID: 34298938 Free PMC article.
-
LAR inhibitory peptide promotes recovery of diaphragm function and multiple forms of respiratory neural circuit plasticity after cervical spinal cord injury.Neurobiol Dis. 2021 Jan;147:105153. doi: 10.1016/j.nbd.2020.105153. Epub 2020 Oct 28. Neurobiol Dis. 2021. PMID: 33127470 Free PMC article.
-
Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors.J Neurosci. 2011 Oct 5;31(40):14051-66. doi: 10.1523/JNEUROSCI.1737-11.2011. J Neurosci. 2011. PMID: 21976490 Free PMC article.
References
-
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M ( 1995) Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron 15: 1375–1381. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W ( 1996) MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma 13: 343–359. - PubMed
-
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH ( 2001) Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 4: 38–43. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB ( 2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
