Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum
- PMID: 12764127
- PMCID: PMC6741072
- DOI: 10.1523/JNEUROSCI.23-10-04378.2003
Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum
Abstract
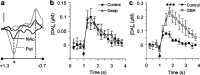
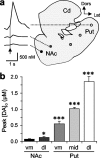
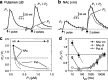
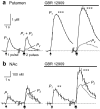
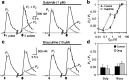
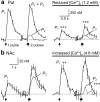
Release of the neuromodulator dopamine (DA) is critical to the control of locomotion, motivation, and reward. However, the probability of DA release is not well understood. Current understanding of neurotransmitter release probability in the CNS is limited to the conventional synaptic amino acid transmitters (e.g., glutamate and GABA). These fast neurotransmitters are released with a repertoire of probabilities according to synapse type, and these probabilities show activity-dependent plasticity according to synapse use. Synapses for neuromodulators such as DA, however, are designed for signaling that diverges temporally and spatially from that for fast neurotransmitters: DA receptors are exclusively metabotropic and at sites that extend to extrasynaptic locations and neighboring synapses. In this study, the release probability of DA was explored in real time in limbicversus motor-associated functional domains of the striatum of a primate (marmoset; Callithrix jacchus) using fast-scan voltammetry at a carbon-fiber microelectrode. We show that the probability of axonal DA release varies with striatal domain. Furthermore, release probability exhibits a short-term, activity-dependent plasticity that ranges from depression to facilitation in motor-through limbic-associated regions, respectively. Rapid plasticity does not result from metabotropic D2-like DA receptor activation or ionotropic GABA(A) receptor effects but is dependent on Ca2+ availability. These data reveal that rapid dynamics in DA release probability will participate in the transmission of the patterns and frequencies encoded by DA neuron action potential discharge. Furthermore, the regional variation in these features indicates that limbic-versus motor-associated DA neurons are permitted to generate diverse DA signals in response to a given firing pattern.
Figures
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A ( 2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252. - PubMed
-
- Atwood HL, Karunanithi S ( 2002) Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci 3: 497–516. - PubMed
-
- Bellingham MC, Walmsley B ( 1999) A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron 23: 159–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
