Crystal structure of a bifunctional aldolase-dehydrogenase: sequestering a reactive and volatile intermediate
- PMID: 12764229
- PMCID: PMC165818
- DOI: 10.1073/pnas.1236794100
Crystal structure of a bifunctional aldolase-dehydrogenase: sequestering a reactive and volatile intermediate
Abstract
The crystal structure of the bifunctional enzyme 4-hydroxy-2-ketovalerate aldolase (DmpG)/acylating acetaldehyde dehydrogenase (DmpF), which is involved in the bacterial degradation of toxic aromatic compounds, has been determined by multiwavelength anomalous dispersion (MAD) techniques and refined to 1.7-A resolution. Structures of the two polypeptides represent a previously unrecognized subclass of metal-dependent aldolases, and of a CoA-dependent dehydrogenase. The structure reveals a mixed state of NAD+ binding to the DmpF protomer. Domain movements associated with cofactor binding in the DmpF protomer may be correlated with channeling and activity at the DmpG protomer. In the presence of NAD+ a 29-A-long sequestered tunnel links the two active sites. Two barriers are visible along the tunnel and suggest control points for the movement of the reactive and volatile acetaldehyde intermediate between the two active sites.
Figures


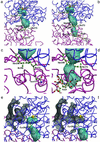
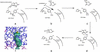
Similar articles
-
Biological channeling of a reactive intermediate in the bifunctional enzyme DmpFG.Biophys J. 2012 Feb 22;102(4):868-77. doi: 10.1016/j.bpj.2012.01.029. Epub 2012 Feb 21. Biophys J. 2012. PMID: 22385858 Free PMC article.
-
Crystallization and preliminary X-ray analysis of dmpFG-encoded 4-hydroxy-2-ketovalerate aldolase--aldehyde dehydrogenase (acylating) from Pseudomonas sp. strain CF600.Acta Crystallogr D Biol Crystallogr. 2001 Apr;57(Pt 4):582-5. doi: 10.1107/s0907444901000439. Acta Crystallogr D Biol Crystallogr. 2001. PMID: 11264589
-
Purification and properties of the physically associated meta-cleavage pathway enzymes 4-hydroxy-2-ketovalerate aldolase and aldehyde dehydrogenase (acylating) from Pseudomonas sp. strain CF600.J Bacteriol. 1993 Jan;175(2):377-85. doi: 10.1128/jb.175.2.377-385.1993. J Bacteriol. 1993. PMID: 8419288 Free PMC article.
-
A shared binding site for NAD+ and coenzyme A in an acetaldehyde dehydrogenase involved in bacterial degradation of aromatic compounds.Biochemistry. 2008 Jul 1;47(26):6870-82. doi: 10.1021/bi800349k. Epub 2008 Jun 7. Biochemistry. 2008. PMID: 18537268
-
The phnIJ genes encoding acetaldehyde dehydrogenase (acylating) and 4-hydroxy-2-oxovalerate aldolase in Pseudomonas sp. DJ77 and their evolutionary implications.Biochem Biophys Res Commun. 1999 Mar 24;256(3):469-73. doi: 10.1006/bbrc.1999.0355. Biochem Biophys Res Commun. 1999. PMID: 10080921
Cited by
-
Investigation into the Mode of Phosphate Activation in the 4-Hydroxy-4-Methyl-2-Oxoglutarate/4-Carboxy-4-Hydroxy-2-Oxoadipate Aldolase from Pseudomonas putida F1.PLoS One. 2016 Oct 14;11(10):e0164556. doi: 10.1371/journal.pone.0164556. eCollection 2016. PLoS One. 2016. PMID: 27741265 Free PMC article.
-
Crystal structure of LeuA from Mycobacterium tuberculosis, a key enzyme in leucine biosynthesis.Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8295-300. doi: 10.1073/pnas.0400820101. Epub 2004 May 24. Proc Natl Acad Sci U S A. 2004. PMID: 15159544 Free PMC article.
-
Binding and channeling of alternative substrates in the enzyme DmpFG: a molecular dynamics study.Biophys J. 2014 Apr 15;106(8):1681-90. doi: 10.1016/j.bpj.2014.03.013. Biophys J. 2014. PMID: 24739167 Free PMC article.
-
Engineering aldolases as biocatalysts.Curr Opin Chem Biol. 2014 Apr;19(100):25-33. doi: 10.1016/j.cbpa.2013.12.010. Epub 2014 Jan 4. Curr Opin Chem Biol. 2014. PMID: 24780276 Free PMC article. Review.
-
Biological channeling of a reactive intermediate in the bifunctional enzyme DmpFG.Biophys J. 2012 Feb 22;102(4):868-77. doi: 10.1016/j.bpj.2012.01.029. Epub 2012 Feb 21. Biophys J. 2012. PMID: 22385858 Free PMC article.
References
-
- Ovadi, J. (1991) J. Theor. Biol. 152, 1–22. - PubMed
-
- Srere, P. A. (1987) Annu. Rev. Biochem. 56, 89–124. - PubMed
-
- Chaudhuri, B. N., Lange, S. C., Myers, R. S., Chittur, S. V., Davisson, V. J. & Smith, J. L. (2001) Structure 9, 987–997. - PubMed
-
- Hyde, C. C., Ahmed, S. A., Padlan, E. A., Miles, E. W. & Davies, D. R. (1988) J. Biol. Chem. 263, 14925–14931. - PubMed
-
- Kim, J. H., Krahn, J. M., Tomchick, D. R., Smith, J. L. & Zalkin, H. (1996) J. Biol. Chem. 271, 15549–15557. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

