Myasthenic syndrome caused by mutation of the SCN4A sodium channel
- PMID: 12766226
- PMCID: PMC165883
- DOI: 10.1073/pnas.1230273100
Myasthenic syndrome caused by mutation of the SCN4A sodium channel
Abstract
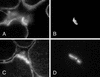
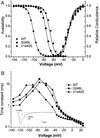
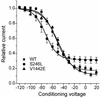
In a myasthenic syndrome associated with fatigable generalized weakness and recurrent attacks of respiratory and bulbar paralysis since birth, nerve stimulation at physiologic rates rapidly decremented the compound muscle action potential. Intercostal muscle studies revealed no abnormality of the resting membrane potential, evoked quantal release, synaptic potentials, acetylcholine receptor channel kinetics, or endplate ultrastructure, but endplate potentials depolarizing the resting potential to -40 mV failed to excite action potentials. Pursuing this clue, we sequenced SCN4A encoding the skeletal muscle sodium channel (Nav1.4) and detected two heteroallelic mutations involving conserved residues not present in 400 normal alleles: S246L in the S4/S5 cytoplasmic linker in domain I, and V1442E in the S3/S4 extracellular linker in domain IV. The genetically engineered V1442E-Na channel expressed in HEK cells shows marked enhancement of fast inactivation close to the resting potential, and enhanced use-dependent inactivation on high-frequency stimulation; S246L is likely a benign polymorphism. The V1442E mutation in SCN4A defines a novel disease mechanism and a novel phenotype with myasthenic features.
Figures




References
-
- Salpeter, M. M. (1987) in Vertebrate Neuromuscular Junctions: General Morphology, Molecular Organization, and Functional Consequences, ed. Salpeter, M. M. (Liss, New York), pp. 1-54.
-
- Flucher, B. E. & Daniels, M. P. (1989) Neuron 3, 163-175. - PubMed
-
- Ruff, R. L. (1996) Acta Physiol. Scand. 156, 159-168. - PubMed
-
- Martin, A. R. (1994) Proc. R. Soc. London B 258, 321-326. - PubMed
-
- Wood, S. J. & Slater, C. P. (2001) Prog. Neurobiol. 64, 393-429. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

