Cytolysis by CCR5-using human immunodeficiency virus type 1 envelope glycoproteins is dependent on membrane fusion and can be inhibited by high levels of CD4 expression
- PMID: 12767984
- PMCID: PMC156190
- DOI: 10.1128/jvi.77.12.6645-6659.2003
Cytolysis by CCR5-using human immunodeficiency virus type 1 envelope glycoproteins is dependent on membrane fusion and can be inhibited by high levels of CD4 expression
Abstract
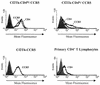
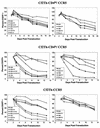
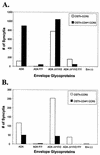
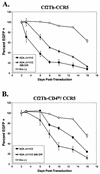
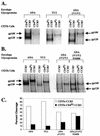
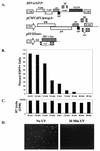
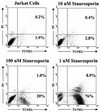
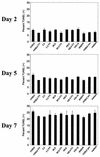
T-tropic (X4) and dualtropic (R5X4) human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins kill primary and immortalized CD4(+) CXCR4(+) T cells by mechanisms involving membrane fusion. However, because much of HIV-1 infection in vivo is mediated by M-tropic (R5) viruses whose envelope glycoproteins use CCR5 as a coreceptor, we tested a panel of R5 and R5X4 envelope glycoproteins for their ability to lyse CCR5(+) target cells. As is the case for CXCR4(+) target cells, HIV-1 envelope glycoproteins expressed by single-round HIV-1 vectors killed transduced CD4(+) CCR5(+) cells in a membrane fusion-dependent manner. Furthermore, a CD4-independent R5 HIV-1 envelope glycoprotein was able to kill CD4-negative target cells expressing CCR5, demonstrating that CD4 is not intrinsically required for the induction of death. Interestingly, high levels of CD4 expression protected cells from lysis and syncytium formation mediated by the HIV-1 envelope glycoproteins. Immunoprecipitation experiments showed that high levels of CD4 coexpression inhibited proteolytic processing of the HIV-1 envelope glycoprotein precursor gp160. This inhibition could be overcome by decreasing the CD4 binding ability of gp120. Studies were also undertaken to investigate the ability of virion-bound HIV-1 envelope glycoproteins to kill primary CD4(+) T cells. However, neither X4 nor R5X4 envelope glycoproteins on noninfectious virions caused death in primary CD4(+) T cells. These results demonstrate that the interaction of CCR5 with R5 HIV-1 envelope glycoproteins capable of inducing membrane fusion leads to cell lysis; overexpression of CD4 can inhibit cell killing by limiting envelope glycoprotein processing.
Figures
Similar articles
-
Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4.J Virol. 1998 Mar;72(3):2509-15. doi: 10.1128/JVI.72.3.2509-2515.1998. J Virol. 1998. PMID: 9499115 Free PMC article.
-
R5 HIV gp120-mediated cellular contacts induce the death of single CCR5-expressing CD4 T cells by a gp41-dependent mechanism.J Leukoc Biol. 2004 Oct;76(4):804-11. doi: 10.1189/jlb.0204100. Epub 2004 Jul 16. J Leukoc Biol. 2004. PMID: 15258189
-
Coreceptor competition for association with CD4 may change the susceptibility of human cells to infection with T-tropic and macrophagetropic isolates of human immunodeficiency virus type 1.J Virol. 2000 Jun;74(11):5016-23. doi: 10.1128/jvi.74.11.5016-5023.2000. J Virol. 2000. PMID: 10799575 Free PMC article.
-
Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
-
Molecular Mechanism of HIV-1 Entry.Trends Microbiol. 2019 Oct;27(10):878-891. doi: 10.1016/j.tim.2019.06.002. Epub 2019 Jun 28. Trends Microbiol. 2019. PMID: 31262533 Free PMC article. Review.
Cited by
-
Movements of HIV-1 genomic RNA-APOBEC3F complexes and PKR reveal cytoplasmic and nuclear PKR defenses and HIV-1 evasion strategies.Virus Res. 2016 Feb 2;213:124-139. doi: 10.1016/j.virusres.2015.11.023. Epub 2015 Nov 25. Virus Res. 2016. PMID: 26626364 Free PMC article.
-
The relationship of CCR5 antagonists to CD4+ T-cell gain: a meta-regression of recent clinical trials in treatment-experienced HIV-infected patients.HIV Clin Trials. 2010 Nov-Dec;11(6):351-8. doi: 10.1310/hct1106-351. HIV Clin Trials. 2010. PMID: 21239363 Free PMC article.
-
Conformational and structural features of HIV-1 gp120 underlying the dual receptor antagonism by cross-reactive neutralizing antibody m18.Biochemistry. 2011 Apr 12;50(14):2756-68. doi: 10.1021/bi101160r. Epub 2011 Mar 18. Biochemistry. 2011. PMID: 21351734 Free PMC article.
-
Virus entry: molecular mechanisms and biomedical applications.Nat Rev Microbiol. 2004 Feb;2(2):109-22. doi: 10.1038/nrmicro817. Nat Rev Microbiol. 2004. PMID: 15043007 Free PMC article. Review.
-
Small CD4 Mimetics Prevent HIV-1 Uninfected Bystander CD4 + T Cell Killing Mediated by Antibody-dependent Cell-mediated Cytotoxicity.EBioMedicine. 2015 Dec 9;3:122-134. doi: 10.1016/j.ebiom.2015.12.004. eCollection 2016 Jan. EBioMedicine. 2015. PMID: 26870823 Free PMC article.
References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Asjo, B., L. Morfeldt-Manson, J. Albert, G. Biberfeld, A. Karlsson, K. Lidman, and E. M. Fenyo. 1986. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet ii:660-662. - PubMed
-
- Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. - PubMed
-
- Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

