Mechanisms of avian retroviral host range extension
- PMID: 12767991
- PMCID: PMC156181
- DOI: 10.1128/jvi.77.12.6709-6719.2003
Mechanisms of avian retroviral host range extension
Abstract
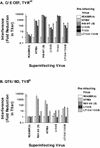
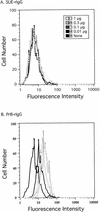
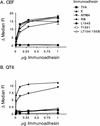
Alpharetroviruses provide a useful system for the study of the molecular mechanisms of host range and receptor interaction. These viruses can be divided into subgroups based on diverse receptor usage due to variability within the two host range determining regions, hr1 and hr2, in their envelope glycoprotein SU (gp85). In previous work, our laboratory described selection from a subgroup B avian sarcoma-leukosis virus of an extended-host-range variant (LT/SI) with two adjacent amino acid substitutions in hr1. This virus retains its ability to use the subgroup BD receptor but can also infect QT6/BD cells, which bear a related subgroup E receptor (R. A. Taplitz and J. M. Coffin, J. Virol 71:7814-7819, 1997). Here, we report further analysis of this unusual variant. First, one (L154S) of the two substitutions is sufficient for host range extension, while the other (T155I) does not alter host range. Second, these mutations extend host range to non-avian cell types, including human, dog, cat, mouse, rat, and hamster. Third, interference experiments imply that the mutants interact efficiently with the subgroup BD receptor and possibly the related subgroup E receptor, but they have another means of entry that is not dependent on these interactions. Fourth, binding studies indicate that the mutant SU proteins retain the ability to interact as monomers with subgroup BD and BDE receptors but only bind the subgroup E receptor in the context of an Env trimer. Further, the mutant SU proteins bind well to chicken cells but do not bind any better than wild-type subgroup B to QT6 or human cells, even though the corresponding viruses are capable of infecting these cells.
Figures




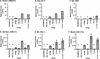
Similar articles
-
Identification of key residues in subgroup A avian leukosis virus envelope determining receptor binding affinity and infectivity of cells expressing chicken or quail Tva receptor.J Virol. 2001 Jan;75(2):726-37. doi: 10.1128/JVI.75.2.726-737.2001. J Virol. 2001. PMID: 11134286 Free PMC article.
-
Selection of an avian retrovirus mutant with extended receptor usage.J Virol. 1997 Oct;71(10):7814-9. doi: 10.1128/JVI.71.10.7814-7819.1997. J Virol. 1997. PMID: 9311868 Free PMC article.
-
Model of the TVA receptor determinants required for efficient infection by subgroup A avian sarcoma and leukosis viruses.J Virol. 2015 Feb;89(4):2136-48. doi: 10.1128/JVI.02339-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473063 Free PMC article.
-
Reverse Engineering Provides Insights on the Evolution of Subgroups A to E Avian Sarcoma and Leukosis Virus Receptor Specificity.Viruses. 2019 May 30;11(6):497. doi: 10.3390/v11060497. Viruses. 2019. PMID: 31151254 Free PMC article. Review.
-
Sequencing the Biology of Entry: The Retroviral env Gene.Curr Top Microbiol Immunol. 2017;407:65-82. doi: 10.1007/82_2017_35. Curr Top Microbiol Immunol. 2017. PMID: 28688086 Free PMC article. Review.
Cited by
-
Heterologous avian system for quantitative analysis of Syncytin-1 interaction with ASCT2 receptor.Retrovirology. 2021 Jun 22;18(1):15. doi: 10.1186/s12977-021-00558-0. Retrovirology. 2021. PMID: 34158079 Free PMC article.
-
Evolution of broad host range in retroviruses leads to cell death mediated by highly cytopathic variants.J Virol. 2006 Jan;80(2):562-70. doi: 10.1128/JVI.80.2.562-570.2006. J Virol. 2006. PMID: 16378958 Free PMC article.
-
The key amino acid sites 199-205, 269, 319, 321 and 324 of ALV-K env contribute to the weaker replication capacity of ALV-K than ALV-A.Retrovirology. 2022 Aug 24;19(1):19. doi: 10.1186/s12977-022-00598-0. Retrovirology. 2022. PMID: 36002842 Free PMC article.
-
Effects of epistasis on infectivity range during host-parasite coevolution.Evolution. 2014 Oct;68(10):2972-82. doi: 10.1111/evo.12479. Epub 2014 Jul 25. Evolution. 2014. PMID: 24957848 Free PMC article.
-
Retroviral host range extension is coupled with Env-activating mutations resulting in receptor-independent entry.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):E5148-E5157. doi: 10.1073/pnas.1704750114. Epub 2017 Jun 12. Proc Natl Acad Sci U S A. 2017. PMID: 28607078 Free PMC article.
References
-
- Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

