Follicular dendritic cell dedifferentiation by treatment with an inhibitor of the lymphotoxin pathway dramatically reduces scrapie susceptibility
- PMID: 12768004
- PMCID: PMC156207
- DOI: 10.1128/jvi.77.12.6845-6854.2003
Follicular dendritic cell dedifferentiation by treatment with an inhibitor of the lymphotoxin pathway dramatically reduces scrapie susceptibility
Abstract
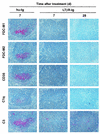
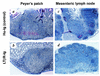
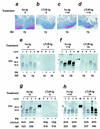
Transmissible spongiform encephalopathies (TSEs) may be acquired peripherally, in which case infectivity usually accumulates in lymphoid tissues before dissemination to the nervous system. Studies of mouse scrapie models have shown that mature follicular dendritic cells (FDCs), expressing the host prion protein (PrP(c)), are critical for replication of infection in lymphoid tissues and subsequent neuroinvasion. Since FDCs require lymphotoxin signals from B lymphocytes to maintain their differentiated state, blockade of this stimulation with a lymphotoxin beta receptor-immunoglobulin fusion protein (LT beta R-Ig) leads to their temporary dedifferentiation. Here, a single treatment with LT beta R-Ig before intraperitoneal scrapie inoculation blocked the early accumulation of infectivity and disease-specific PrP (PrP(Sc)) within the spleen and substantially reduced disease susceptibility. These effects coincided with an absence of FDCs in the spleen for ca. 28 days after treatment. Although the period of FDC dedifferentiation was extended to at least 49 days by consecutive LT beta R-Ig treatments, this had little added protective benefit after injection with a moderate dose of scrapie. We also demonstrate that mature FDCs are critical for the transmission of scrapie from the gastrointestinal tract. Treatment with LT beta R-Ig before oral scrapie inoculation blocked PrP(Sc) accumulation in Peyer's patches and mesenteric lymph nodes and prevented neuroinvasion. However, treatment 14 days after oral inoculation did not affect survival time or susceptibility, suggesting that infectivity may have already spread to the peripheral nervous system. Although manipulation of FDCs may offer a potential approach for early intervention in peripherally acquired TSEs, these data suggest that the duration of the treatment window may vary widely depending on the route of exposure.
Figures



References
-
- Andreoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J.-M. Elsen, and F. Lantier. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. - PubMed
-
- Beekes, M., and P. A. McBride. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 278:181-184. - PubMed
-
- Beringue, V., M. Demoy, C. I. Lasmezas, B. Gouritin, C. Weingarten, J.-P. Deslys, J.-P. Adreux, P. Couvreur, and D. Dormont. 2000. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 190:495-502. - PubMed
-
- Bolton, D. C., M. P. McKinley, and S. B. Prusiner. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309-1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

