Human immunodeficiency virus type 1 envelope-mediated neuronal death: uncoupling of viral replication and neurotoxicity
- PMID: 12768009
- PMCID: PMC156161
- DOI: 10.1128/jvi.77.12.6899-6912.2003
Human immunodeficiency virus type 1 envelope-mediated neuronal death: uncoupling of viral replication and neurotoxicity
Abstract
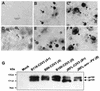
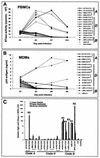
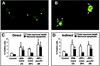
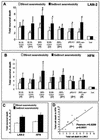
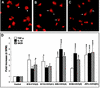
Although brain tissue from patients with human immunodeficiency virus (HIV) and/or AIDS is consistently infected by HIV type 1 (HIV-1), only 20 to 30% of patients exhibit clinical or neuropathological evidence of brain injury. Extensive HIV-1 sequence diversity is present in the brain, which may account in part for the variability in the occurrence of HIV-induced brain disease. Neurological injury caused by HIV-1 is mediated directly by neurotoxic viral proteins or indirectly through excess production of host molecules by infected or activated glial cells. To elucidate the relationship between HIV-1 infection and neuronal death, we examined the neurotoxic effects of supernatants from human 293T cells or macrophages expressing recombinant HIV-1 virions or gp120 proteins containing the V1V3 or C2V3 envelope region from non-clade B, brain-derived HIV-1 sequences. Neurotoxicity was measured separately as apoptosis or total neuronal death, with apoptosis representing 30 to 80% of the total neuron death observed, depending on the individual virus. In addition, neurotoxicity was dependent on expression of HIV-1 gp120 and could be blocked by anti-gp120 antibodies, as well as by antibodies to the human CCR5 and CXCR4 chemokine receptors. Despite extensive sequence diversity in the recombinant envelope region (V1V3 or C2V3), there was limited variation in the neurotoxicity induced by supernatants from transfected 293T cells. Conversely, supernatants from infected macrophages caused a broader range of neurotoxicity levels that depended on each virus and was independent of the replicative ability of the virus. These findings underscore the importance of HIV-1 envelope protein expression in neurotoxic pathways associated with HIV-induced brain disease and highlight the envelope as a target for neuroprotective therapeutic interventions.
Figures


Similar articles
-
Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients.J Virol. 1998 Nov;72(11):9045-53. doi: 10.1128/JVI.72.11.9045-9053.1998. J Virol. 1998. PMID: 9765449 Free PMC article.
-
HIV-1 gp120 proteins and gp160 peptides are toxic to brain endothelial cells and neurons: possible pathway for HIV entry into the brain and HIV-associated dementia.J Neuropathol Exp Neurol. 2002 Nov;61(11):992-1000. doi: 10.1093/jnen/61.11.992. J Neuropathol Exp Neurol. 2002. PMID: 12430716
-
Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis.Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):8212-6. doi: 10.1073/pnas.96.14.8212. Proc Natl Acad Sci U S A. 1999. PMID: 10393974 Free PMC article.
-
Comparative neurovirulence in lentiviral infections: The roles of viral molecular diversity and select proteases.J Neurovirol. 2004;10 Suppl 1:113-7. doi: 10.1080/753312762. J Neurovirol. 2004. PMID: 14982749 Review.
-
Cell death in HIV dementia.Cell Death Differ. 2005 Aug;12 Suppl 1:893-904. doi: 10.1038/sj.cdd.4401577. Cell Death Differ. 2005. PMID: 15761472 Review.
Cited by
-
Differences in the fitness of two diverse wild-type human immunodeficiency virus type 1 isolates are related to the efficiency of cell binding and entry.J Virol. 2005 Jun;79(11):7121-34. doi: 10.1128/JVI.79.11.7121-7134.2005. J Virol. 2005. PMID: 15890952 Free PMC article.
-
Apoptosis of uninfected cells induced by HIV envelope glycoproteins.Retrovirology. 2004 Jun 23;1:12. doi: 10.1186/1742-4690-1-12. Retrovirology. 2004. PMID: 15214962 Free PMC article. Review.
-
HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration.J Neurosci. 2007 Apr 4;27(14):3703-11. doi: 10.1523/JNEUROSCI.5522-06.2007. J Neurosci. 2007. PMID: 17409234 Free PMC article.
-
Molecular Signatures of HIV-1 Envelope Associated with HIV-Associated Neurocognitive Disorders.Curr HIV/AIDS Rep. 2018 Feb;15(1):72-83. doi: 10.1007/s11904-018-0374-3. Curr HIV/AIDS Rep. 2018. PMID: 29460224 Review.
-
Human immunodeficiency virus type 1 genetic diversity in the nervous system: evolutionary epiphenomenon or disease determinant?J Neurovirol. 2005 Apr;11(2):107-28. doi: 10.1080/13550280590922838. J Neurovirol. 2005. PMID: 16036790 Review.
References
-
- Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retrovir. 10:371-381. - PubMed
-
- Adle-Biassette, H., F. Chretien, L. Wingertsmann, C. Hery, T. Ereau, F. Scaravilli, M. Tardieu, and F. Gray. 1999. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 25:123-133. - PubMed
-
- Adle-Biassette, H., Y. Levy, M. Colombel, F. Poron, S. Natchev, C. Keohane, and F. Gray. 1995. Neuronal apoptosis in HIV infection in adults. Neuropathol. Appl. Neurobiol. 21:218-227. - PubMed
-
- Bagetta, G., M. T. Corasaniti, L. Berliocchi, M. Navarra, A. Finazzi-Agro, and G. Nistico. 1995. HIV-1 gp120 produces DNA fragmentation in the cerebral cortex of rat. Biochem. Biophys. Res. Commun. 211:130-136. - PubMed
-
- Bagetta, G., M. T. Corasaniti, W. Malorni, G. Rainaldi, L. Berliocchi, A. Finazzi-Agro, and G. Nistico. 1996. The HIV-1 gp120 causes ultrastructural changes typical of apoptosis in the rat cerebral cortex. Neuroreport 7:1722-1724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

