Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity
- PMID: 12768012
- PMCID: PMC156179
- DOI: 10.1128/jvi.77.12.6931-6945.2003
Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity
Abstract
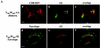
Here, we report that human immunodeficiency virus type 1 (HIV-1) Env glycoprotein is located mainly in the trans-Golgi network (TGN) due to determinants present in the cytoplasmic domain of the transmembrane gp41 glycoprotein (TMgp41). Internalization assays demonstrated that Env present at the cell surface returns to the TGN. We found that the cytoplasmic domain of TMgp41 binds to TIP47, a protein required for the transport of mannose-6-phosphate receptors from endosomes to the TGN. Overexpression of a mutant of TIP47 affected the transport of Env from endosomes to the TGN. Retrograde transport of Env to the TGN requires a Y(802)W(803) diaromatic motif present in the TMgp41 cytoplasmic domain. Mutation of this motif abolished both targeting to the TGN as well as interaction with TIP47. These data support the view that binding of TIP47 to HIV-1 Env facilitates its delivery to the TGN. Lastly, we show that virus mutated in the Y(802)W(803) motif is poorly infectious and presents a defect in Env incorporation, supporting a model in which retrograde transport of Env is implicated in the optimization of fully infectious HIV-1 production.
Figures
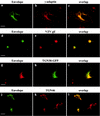

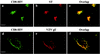
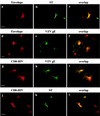
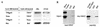
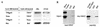



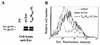

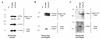
References
-
- Barbero, P., E. Buell, S. Zulley, and S. R. Pfeffer. 2001. Tip47 is not a component of lipid droplets. J. Biol. Chem. 276:24348-24351. - PubMed
-
- Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. - PMC - PubMed
-
- Blom, J., C. Nielsen, and J. M. Rhodes. 1993. An ultrastructural study of HIV-infected human dendritic cells and monocytes/macrophages. APMIS 101:672-680. - PubMed
-
- Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

