Human cytomegalovirus infection inhibits tumor necrosis factor alpha (TNF-alpha) signaling by targeting the 55-kilodalton TNF-alpha receptor
- PMID: 12768019
- PMCID: PMC156201
- DOI: 10.1128/jvi.77.12.7007-7016.2003
Human cytomegalovirus infection inhibits tumor necrosis factor alpha (TNF-alpha) signaling by targeting the 55-kilodalton TNF-alpha receptor
Abstract
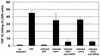
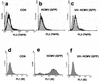
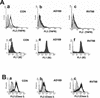
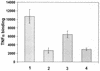
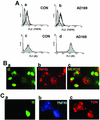
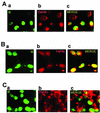
Infection with human cytomegalovirus (HCMV) results in complex interactions between viral and cellular factors which perturb many cellular functions. HCMV is known to target the cell cycle, cellular transcription, and immunoregulation, and it is believed that this optimizes the cellular environment for viral DNA replication during productive infection or during carriage in the latently infected host. Here, we show that HCMV infection also prevents external signaling to the cell by disrupting the function of TNFRI, the 55-kDa receptor for tumor necrosis factor alpha (TNF-alpha), one of the receptors for a potent cytokine involved in eliciting a wide spectrum of cellular responses, including antiviral responses. HCMV infection of fully permissive differentiated monocytic cell lines and U373 cells resulted in a reduction in cell surface expression of TNFRI. The reduction appeared to be due to relocalization of TNFRI from the cell surface and was reflected in the elimination of TNF-alpha-induced Jun kinase activity. Analysis of specific phases of infection suggested that viral early gene products were responsible for this relocalization. However, a mutant HCMV in which all viral gene products known to be involved in down-regulation of major histocompatibility complex (MHC) class I were deleted still resulted in relocalization of TNFRI. Consequently, TNFRI relocalization by HCMV appears to be mediated by a novel viral early function not involved in down-regulation of cell surface MHC class I expression. We suggest that upon infection, HCMV isolates the cell from host-mediated signals, forcing the cell to respond only to virus-specific signals which optimize the cell for virus production and effect proviral responses from bystander cells.
Figures


References
-
- Alcami, A., A. Khanna, N. L. Paul, and G. L. Smith. 1999. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J. Gen. Virol. 80:949-959. - PubMed
-
- Barnes, P. D., and J. E. Grundy. 1992. Down-regulation of the class I HLA heterodimer and beta 2-microglobulin on the surface of cells infected with cytomegalovirus. J. Gen. Virol. 73:2395-2403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

