Generation of transduction-competent retroviral vectors by infection with a single hybrid vaccinia virus
- PMID: 12768020
- PMCID: PMC156191
- DOI: 10.1128/jvi.77.12.7017-7025.2003
Generation of transduction-competent retroviral vectors by infection with a single hybrid vaccinia virus
Abstract
Recombinant vaccinia viruses that express defective retroviral vectors upon a single infection event in normal host cells were constructed. The gag-pol and envelope genes and a retroviral vector unit were inserted as vaccinia virus promoter-controlled transcription units at three separate loci. The triple recombinant virus was used to infect such diverse cell types as monkey and rabbit kidney, human lung, and primary chicken cells, resulting in the production of transduction-competent defective retroviral vectors. Infection of Chinese hamster ovary cells, which are nonpermissive for vaccinia virus replication, also resulted in production of retroviral vectors and secondary permanent transduction of the host cells. Since vaccinia virus supports the expression of cytotoxic proteins, the vesicular stomatitis virus G glycoprotein could be chosen as the envelope allowing a broad host range of transduction. Functionality of particles was monitored by expression of the green fluorescent protein in transduced 3T3 cell clones. This is the first description of a single chimeric virus encoding and releasing functional retroviral vectors, providing proof of principle of the new concept. No replication-competent retrovirus was detectable by sensitive reverse transcriptase assays. Since vaccinia virus has a broad host range, is extremely robust, and can be obtained at high titers and safe nonreplicating vaccinia virus strains are available, the hybrid system may open new perspectives for gene delivery.
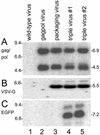
Figures






References
-
- Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. - PMC - PubMed
-
- Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. - PubMed
-
- Feng, M., W. H. Jackson, Jr., C. K. Goldman, C. Rancourt, M. Wang, S. K. Dusing, G. Siegal, and D. T. Curiel. 1997. Stable in vivo gene transduction via a novel adenoviral/retroviral chimeric vector. Nat. Biotechnol. 15:866-870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

