Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice
- PMID: 12768022
- PMCID: PMC156185
- DOI: 10.1128/jvi.77.12.7034-7040.2003
Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice
Abstract
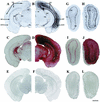
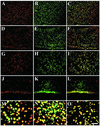
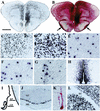
Inherited metabolic disorders that affect the central nervous system typically result in pathology throughout the brain; thus, gene therapy strategies need to achieve widespread delivery. We previously found that although intraventricular injection of the neonatal mouse brain with adeno-associated virus serotype 2 (AAV2) results in dispersed gene delivery, many brain structures were poorly transduced. This limitation may be overcome by using different AAV serotypes because the capsid proteins use different cellular receptors for entry, which may allow enhanced global targeting of the brain. We tested this with AAV1 and AAV5 vectors. AAV5 showed very limited brain transduction after neonatal injection, even though it has different transduction patterns than AAV2 in adult brain injections. In contrast, AAV1 vectors, which have not been tested in the brain, showed robust widespread transduction. Complementary patterns of transduction between AAV1 and AAV2 were established and maintained in the adult brain after neonatal injection. In the majority of structures, AAV1 transduced many more cells than AAV2. Both vectors transduced mostly neurons, indicating that differential expression of receptors on the surfaces of neurons occurs in the developing brain. The number of cells positive for a vector-encoded secreted enzyme (beta-glucuronidase) was notably greater and more widespread in AAV1-injected brains. A comprehensive analysis of AAV1-treated brains from beta-glucuronidase-deficient mice (mucopolysaccharidosis type VII) showed complete reversal of pathology in all areas of the brain for at least 1 year, demonstrating that the combination of this serotype and experimental strategy is therapeutically effective for treating global neurometabolic disorders.
Figures

References
-
- Alisky, J. M., S. M. Hughes, S. L. Sauter, D. Jolly, T. W. Dubensky, P. D. Staber, J. A. Chiorini, and B. L. Davidson. 2000. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport 1:2669-2673. - PubMed
-
- Auricchio, A., G. Kobinger, V. Anand, M. Hildinger, E. O'Connor, A. M. Maguire, J. M. Wilson, and J. Bennett. 2001. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 10:3075-3081. - PubMed
-
- Barthel, L. K., and P. A. Raymond. 2000. In situ hybridization studies of retinal neurons. Methods Enzymol. 316:579-590. - PubMed
-
- Bartlett, J. S., R. J. Samulski, and T. J. McCown. 1998. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum. Gene Ther. 9:1181-1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

