Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes
- PMID: 12768024
- PMCID: PMC156204
- DOI: 10.1128/jvi.77.12.7048-7057.2003
Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes
Abstract
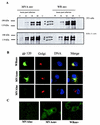
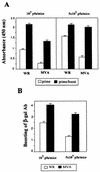
Vaccines that elicit systemic and mucosal immune responses should be the choice to control human immunodeficiency virus (HIV) infections. We have previously shown that prime-boost immunizations with influenza virus Env and vaccinia virus (VV) WR Env recombinants induced an enhanced systemic CD8(+) T-cell response against HIV-1 Env antigen. In this report, we analyzed in BALB/c mice after priming with influenza virus Env the ability of two VV recombinants expressing HIV-1 Env B (VV WR Env and the highly attenuated modified VV Ankara [MVA] Env) to boost cellular immune responses in the spleen and in the lymph nodes draining the genital and rectal tracts. Groups of mice were primed by the intranasal route with 10(4) PFU of influenza virus Env and boosted 14 days later by the intraperitoneal or intranasal route with 10(7) PFU of MVA Env or VV WR Env, while the control group received two immunizations with influenza virus Env. We found that the combined immunization (Flu/VV) increased more than 60 times the number of gamma interferon-specific CD8(+) T cells compared to the Flu/Flu scheme. Significantly, boosting with MVA Env by the intraperitoneal route induced a response 1.25 or 2.5 times (spleen or genital lymph nodes) higher with respect to that found after the boost with VV WR Env. Mice with an enhanced CD8(+) T-cell response also had an increased Th1/Th2 ratio, evaluated by the cytokine pattern secreted following in vitro restimulation with gp160 protein and by the specific immunoglobulin G2a (IgG2a)/IgG1 ratio in serum. By the intranasal route recombinant WR Env booster gave a more efficient immune response (10 and 1.3 times in spleen and genital lymph nodes, respectively) than recombinant MVA Env. However, the scheme influenza virus Env/MVA Env increased four times the response in the spleen, giving a low but significant response in the genital lymph nodes compared with a single intranasal immunization with MVA Env. These results demonstrate that the combination Flu/MVA in prime-booster immunization regimens is an effective vaccination approach to generate cellular immune responses to HIV antigens at sites critical for protective responses.
Figures

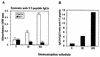

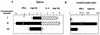

Similar articles
-
Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule.J Immunol. 2004 May 15;172(10):6209-20. doi: 10.4049/jimmunol.172.10.6209. J Immunol. 2004. PMID: 15128809
-
Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma.Virus Res. 2006 Mar;116(1-2):11-20. doi: 10.1016/j.virusres.2005.08.008. Epub 2005 Oct 7. Virus Res. 2006. PMID: 16214252
-
Enhanced CD8+ T cell immune response against a V3 loop multi-epitope polypeptide (TAB13) of HIV-1 Env after priming with purified fusion protein and booster with modified vaccinia virus Ankara (MVA-TAB) recombinant: a comparison of humoral and cellular immune responses with the vaccinia virus Western Reserve (WR) vector.Vaccine. 2001 Dec 12;20(5-6):961-71. doi: 10.1016/s0264-410x(01)00389-9. Vaccine. 2001. PMID: 11738764
-
Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens.Histol Histopathol. 2001 Apr;16(2):655-67. doi: 10.14670/HH-16.655. Histol Histopathol. 2001. PMID: 11332721 Review.
-
Recombinant poxviruses as mucosal vaccine vectors.J Gen Virol. 2005 Nov;86(Pt 11):2925-2936. doi: 10.1099/vir.0.81181-0. J Gen Virol. 2005. PMID: 16227213 Review.
Cited by
-
Selective induction of host genes by MVA-B, a candidate vaccine against HIV/AIDS.J Virol. 2010 Aug;84(16):8141-52. doi: 10.1128/JVI.00749-10. Epub 2010 Jun 9. J Virol. 2010. PMID: 20534857 Free PMC article.
-
Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential?Curr Opin Biotechnol. 2004 Dec;15(6):506-12. doi: 10.1016/j.copbio.2004.09.001. Curr Opin Biotechnol. 2004. PMID: 15560976 Free PMC article. Review.
-
Vaginal immunization to elicit primary T-cell activation and dissemination.PLoS One. 2013 Dec 5;8(12):e80545. doi: 10.1371/journal.pone.0080545. eCollection 2013. PLoS One. 2013. PMID: 24349003 Free PMC article.
-
Induction of Potent and Long-Lived Antibody and Cellular Immune Responses in the Genitorectal Mucosa Could be the Critical Determinant of HIV Vaccine Efficacy.Front Immunol. 2014 May 8;5:202. doi: 10.3389/fimmu.2014.00202. eCollection 2014. Front Immunol. 2014. PMID: 24847327 Free PMC article. Review.
-
Inclusion of a CRF01_AE HIV envelope protein boost with a DNA/MVA prime-boost vaccine: Impact on humoral and cellular immunogenicity and viral load reduction after SHIV-E challenge.Vaccine. 2012 Feb 27;30(10):1830-40. doi: 10.1016/j.vaccine.2011.12.131. Epub 2012 Jan 9. Vaccine. 2012. PMID: 22234262 Free PMC article.
References
-
- Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified VV Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. - PMC - PubMed
-
- Belyakov, I. M., L. S. Wyatt, J. D. Ahlers, P. L. Earl, C. D. Pendleton, B. L. Kelsall, W. Strober, B. Moss, and J. A. Berzofsky. 1998. Induction of a mucosal cytotoxic T-limphocyte response by intrarectal immunization with a replication-deficient recombinant VV expressing human immunodeficiency virus 89.6 envelope protein. J. Virol. 72:8264-8272. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

