Basic residues of the helix six domain of influenza virus M1 involved in nuclear translocation of M1 can be replaced by PTAP and YPDL late assembly domain motifs
- PMID: 12768027
- PMCID: PMC156155
- DOI: 10.1128/jvi.77.12.7078-7092.2003
Basic residues of the helix six domain of influenza virus M1 involved in nuclear translocation of M1 can be replaced by PTAP and YPDL late assembly domain motifs
Retraction in
-
Retraction.J Virol. 2006 Oct;80(20):10289. doi: 10.1128/JVI.01632-06. J Virol. 2006. PMID: 17005709 Free PMC article. No abstract available.
Abstract
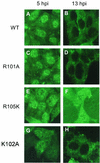
Influenza type A virus matrix (M1) protein possesses multiple functional motifs in the helix 6 (H6) domain (amino acids 91 to 105), including nuclear localization signal (NLS) (101-RKLKR-105) involved in translocating M1 from the cytoplasm into the nucleus. To determine the role of the NLS motif in the influenza virus life cycle, we mutated these and the neighboring sequences by site-directed mutagenesis, and influenza virus mutants were generated by reverse genetics. Our results show that infectious viruses were rescued by reverse genetics from all single alanine mutations of amino acids in the H6 domain and the neighboring region except in three positions (K104A and R105A within the NLS motif and E106A in loop 6 outside the NLS motif). Among the rescued mutant viruses, R101A and R105K exhibited reduced growth and small-plaque morphology, and all other mutant viruses showed the wild-type phenotype. On the other hand, three single mutations (K104A, K105A, and E106A) and three double mutations (R101A/K102A, K104A/K105A, and K102A/R105A) failed to generate infectious virus. Deletion (Delta YRKL) or mutation (4A) of YRKL also abolished generation of infectious virus. However, replacement of the YRKL motif with PTAP or YPDL as well as insertion of PTAP after 4A mutation yielded infectious viruses with the wild-type phenotype. Furthermore, mutant M1 proteins (R101A/K102A, Delta YRKL, 4A, PTAP, 4A+PTAP, and YPDL) when expressed alone from cloned cDNAs were only cytoplasmic, whereas the wild-type M1 expressed alone was both nuclear and cytoplasmic as expected. These results show that the nuclear translocation function provided by the positively charged residues within the NLS motif does not play a critical role in influenza virus replication. Furthermore, these sequences of H6 domain can be replaced by late (L) domain motifs and therefore may provide a function similar to that of the L domains of other negative-strand RNA and retroviruses.
Figures









References
-
- Allen, H., J. McCauley, M. Waterfield, and M. J. Gething. 1980. Influenza virus RNA segment 7 has the coding capacity for two polypeptides. Virology 107:548-551. - PubMed
-
- Arzt, S., F. Baudin, A. Barge, P. Timmins, W. P. Burmeister, and R. W. H. Ruigrok. 2001. Combined results from solution studies on intact influenza virus M1 protein and from a new crystal form of its N-terminal domain show that M1 is an elongated monomer. Virology 279:439-446. - PubMed
-
- Barman, S., L. Adhikary, Y. Kawaoka, and D. P. Nayak. 2003. Influenza A virus hemagglutinin containing basolateral localization signal does not alter the apical budding of a recombinant influenza A virus in polarized MDCK cells. Virology 305:138-152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

