The effect of lipid demixing on the electrostatic interaction of planar membranes across a salt solution
- PMID: 12770879
- PMCID: PMC1302955
- DOI: 10.1016/S0006-3495(03)75101-7
The effect of lipid demixing on the electrostatic interaction of planar membranes across a salt solution
Abstract
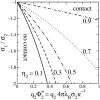
We study the effect of lipid demixing on the electrostatic interaction of two oppositely-charged membranes in solution, modeled here as an incompressible two-dimensional fluid mixture of neutral and charged mobile lipids. We calculate, within linear and nonlinear Poisson-Boltzmann theory, the membrane separation at which the net electrostatic force between the membranes vanishes, for a variety of different system parameters. According to Parsegian and Gingell, contact between oppositely-charged surfaces in an electrolyte is possible only if the two surfaces have exactly the same charge density (sigma(1) = -sigma(2)). If this condition is not fulfilled, the surfaces can repel each other, even though they are oppositely charged. In our model of a membrane, the lipidic charge distribution on the membrane surface is not homogeneous and frozen, but the lipids are allowed to freely move within the plane of the membrane. We show that lipid demixing allows contact between membranes even if there is a certain charge mismatch, /sigma(1)/ not equal /sigma(2)/, and that in certain limiting cases, contact is always possible, regardless of the value of sigma(1)/sigma(2) (if sigma(1)/sigma(2) < 0). We furthermore find that of the two interacting membranes, only one membrane shows a major rearrangement of lipids, whereas the other remains in exactly the same state it has in isolation and that, at zero-disjoining pressure, the electrostatic mean-field potential between the membranes follows a Gouy-Chapman potential from the more strongly charged membrane up to the point of the other, more weakly charged membrane.
Figures




Similar articles
-
Lipid demixing and protein-protein interactions in the adsorption of charged proteins on mixed membranes.Biophys J. 2000 Oct;79(4):1747-60. doi: 10.1016/S0006-3495(00)76427-7. Biophys J. 2000. PMID: 11023883 Free PMC article.
-
Protein diffusion on charged membranes: a dynamic mean-field model describes time evolution and lipid reorganization.Biophys J. 2008 Apr 1;94(7):2580-97. doi: 10.1529/biophysj.107.120667. Epub 2007 Dec 7. Biophys J. 2008. PMID: 18065451 Free PMC article.
-
Domain formation induced by the adsorption of charged proteins on mixed lipid membranes.Biophys J. 2005 Mar;88(3):1702-14. doi: 10.1529/biophysj.104.048132. Epub 2004 Dec 30. Biophys J. 2005. PMID: 15626713 Free PMC article.
-
Roles of bilayer material properties in function and distribution of membrane proteins.Annu Rev Biophys Biomol Struct. 2006;35:177-98. doi: 10.1146/annurev.biophys.35.040405.102022. Annu Rev Biophys Biomol Struct. 2006. PMID: 16689633 Review.
-
The electrostatics of lipid surfaces.Chem Phys Lipids. 1999 Aug;101(1):3-35. doi: 10.1016/s0009-3084(99)00052-3. Chem Phys Lipids. 1999. PMID: 10810922 Review.
Cited by
-
Interaction of poly(L-lysines) with negatively charged membranes: an FT-IR and DSC study.Eur Biophys J. 2007 Apr;36(4-5):437-50. doi: 10.1007/s00249-006-0080-8. Epub 2006 Aug 16. Eur Biophys J. 2007. PMID: 16912868
-
Natural lipid extracts and biomembrane-mimicking lipid compositions are disposed to form nonlamellar phases, and they release DNA from lipoplexes most efficiently.Biochim Biophys Acta. 2007 Oct;1768(10):2373-82. doi: 10.1016/j.bbamem.2007.04.026. Epub 2007 May 5. Biochim Biophys Acta. 2007. PMID: 17559800 Free PMC article.
-
Effect of sodium chloride on a lipid bilayer.Biophys J. 2003 Sep;85(3):1647-55. doi: 10.1016/S0006-3495(03)74594-9. Biophys J. 2003. PMID: 12944279 Free PMC article.
-
Increased concentration of polyvalent phospholipids in the adsorption domain of a charged protein.Biophys J. 2004 Apr;86(4):2165-78. doi: 10.1016/S0006-3495(04)74276-9. Biophys J. 2004. PMID: 15041657 Free PMC article.
-
Aberrant ER-mitochondria communication is a common pathomechanism in mitochondrial disease.Cell Death Dis. 2024 Jun 10;15(6):405. doi: 10.1038/s41419-024-06781-9. Cell Death Dis. 2024. PMID: 38858390 Free PMC article.
References
-
- Almeida, P. F. F., and W. L. C. Vaz. 1995. Lateral diffusion in membranes. In Structure and Dynamics of Membranes: From Cells to Vesicles. R. Lipowski and E. Sackmann, editors. Elsevier, Amsterdam, the Netherlands. pp.305–357.
-
- Andelman, D. 1995. Electrostatic properties of membranes: the Poisson-Boltzmann theory. In Structure and Dynamics of Membranes. R. Lipowsky and E. Sackmann, editors. North Holland, Amsterdam, the Netherlands. p.603.
-
- Aranda-Espinoza, H., Y. Chen, N. Dan, T. Lubensky, P. Nelson, L. Ramos, and D. Weitz. 1999. Electrostatic repulsion of positively charged vesicles and negatively charged objects. Science. 285:394–397. - PubMed
-
- Arora, A., M. Esmann, and D. Marsh. 1989. Selectivity of lipid-protein interactions with trypsinized NA,K-ATPase studied by spin-label ESR. Biochim. Biophys. Acta. 1371:163–167. - PubMed
-
- Bell, G. I. 1988. Cell adhesion. In Physical Basis of Cell-Cell Adhesion. P. Bongrand, editor. CRC Press, Boca Raton, FL. p.227.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

