Electric-field dependent decays of two spectroscopically different M-states of photosensory rhodopsin II from Natronobacterium pharaonis
- PMID: 12770892
- PMCID: PMC1302968
- DOI: 10.1016/S0006-3495(03)75114-5
Electric-field dependent decays of two spectroscopically different M-states of photosensory rhodopsin II from Natronobacterium pharaonis
Abstract
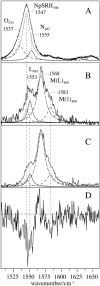
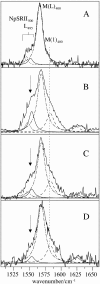
Sensory rhodopsin II (NpSRII) from Natronobacterium pharaonis was studied by resonance Raman (RR) spectroscopic techniques. Using gated 413-nm excitation, time-resolved RR measurements of the solubilized photoreceptor were carried out to probe the photocycle intermediates that are formed in the submillisecond time range. For the first time, two M-like intermediates were identified on the basis of their C=C stretching bands at 1568 and 1583 cm(-1), corresponding to the early M(L)(400) state with a lifetime of 30 micro s and the subsequent M(1)(400) state with a lifetime of 2 ms, respectively. The unusually high C=C stretching frequency of M(1)(400) has been attributed to an unprotonated retinal Schiff base in a largely hydrophobic environment, implying that the M(L)(400) --> M(1)(400) transition is associated with protein structural changes in the vicinity of the chromophore binding pocket. Time-resolved surface enhanced resonance Raman experiments of NpSRII electrostatically bound onto a rotating Ag electrode reveal that the photoreceptor runs through the photocycle also in the immobilized state. Surface enhanced resonance Raman spectra are very similar to the RR spectra of the solubilized protein, ruling out adsorption-induced structural changes in the retinal binding pocket. The photocycle kinetics, however, is sensitively affected by the electrode potential such that at 0.0 V (versus Ag/AgCl) the decay times of M(L)(400) and M(1)(400) are drastically slowed down. Upon decreasing the potential to -0.4 V, that corresponds to a decrease of the interfacial potential drop and thus of the electric field strength at the protein binding site, the photocycle kinetics becomes similar to that of NpSRII in solution. The electric-field dependence of the protein structural changes associated with the M-state transitions, which in the present spectroscopic work is revealed on a molecular level, appears to be related to the electric-field control of bacteriorhodopsin's photocycle, which has been shown to be of functional relevance.
Figures

References
-
- Althaus, T., W. Eisfeld, R. Lohrmann, and M. Stockburger. 1995. Application of Raman spectroscopy to retinal proteins. Isr. J. Chem. 35:227–251.
-
- Bamberg, E., and A. Fahr. 1980. Photocurrents induced on black lipid membranes by purple membranes: a method for reconstitution and a kinetic study of the photocurrents. Ann. N. Y. Acad. Sci. 358:324–327. - PubMed
-
- Bergo, V., E. N. Spudich, K. L. Scott, J. L. Spudich, and K. J. Rothschild. 2000. FTIR analysis of the SII540 intermediate of sensory rhodopsin II: Asp73 is the Schiff base proton acceptor. Biochemistry. 39:2823–2830. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

