Nonresonant confocal Raman imaging of DNA and protein distribution in apoptotic cells
- PMID: 12770902
- PMCID: PMC1302978
- DOI: 10.1016/S0006-3495(03)75124-8
Nonresonant confocal Raman imaging of DNA and protein distribution in apoptotic cells
Abstract
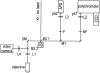
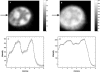
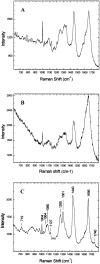
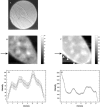
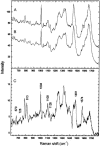
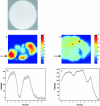
Nonresonant confocal Raman imaging has been used to map the DNA and the protein distributions in individual single human cells. The images are obtained on an improved homebuilt confocal Raman microscope. After statistical analysis, using singular value decomposition, the Raman images are reconstructed from the spectra covering the fingerprint region. The data are obtained at a step interval of approximately 250 nm and cover a field from 8- to 15- micro m square in size. Dwell times at each pixel are between 0.5 and 2 s, depending on the nature and the state of the cell under investigation. High quality nonresonant Raman images can only be obtained under these conditions using continuous wave high laser powers between 60 and 120 mW. We will present evidence that these laser powers can still safely be used to recover the chemical distributions in fixed cells. The developed Raman imaging method is used to image directly, i.e., without prior labeling, the nucleotide condensation and the protein distribution in the so-called nuclear fragments of apoptotic HeLa cells. In the control (nonapoptotic) HeLa cells, we show, for the first time by Raman microspectroscopy, the presence of the RNA in a cell nucleus.
Figures

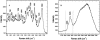



References
-
- Arikan, S., H. S. Sands, R. G. Rodway, and D. N. Batchelder. 2002. Raman spectroscopy and imaging of beta-carotene in live corpus luteum cells. Anim. Reprod. Sci. 71:249–266. - PubMed
-
- Biggiogera, M., M. G. Bottone, and C. Pellicciari. 1998. Nuclear RNA is extruded from apoptotic cells. J. Histochem. Cytochem. 46:999–1005. - PubMed
-
- Byassee, T. A., W. C. Chan, and S. Nie. 2000. Probing single molecules in single living cells. Anal. Chem. 72:5606–5611. - PubMed
-
- Cheng, J.-X., A. Volkmer, L. D. Book, and X. S. Xie. 2001. An epi-detected anti-Stokes Raman scattering (E-CARS) microscope with high spectral resolution and high sensitivity. J. Phys. Chem. B. 105:1277–1280.
-
- Cheng, J.-X., A. Volkmer, L. D. Book, and X. S. Xie. 2002. Multiplex coherent anti-Stokes Raman scattering microspectroscopy and study of lipid vesicles. J. Phys. Chem. B. 106:8493–8498.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

