Selective cell targeting with light-absorbing microparticles and nanoparticles
- PMID: 12770906
- PMCID: PMC1302982
- DOI: 10.1016/S0006-3495(03)75128-5
Selective cell targeting with light-absorbing microparticles and nanoparticles
Abstract
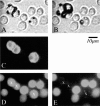
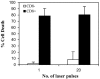
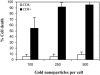
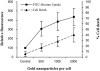
We describe a new method for selective cell targeting based on the use of light-absorbing microparticles and nanoparticles that are heated by short laser pulses to create highly localized cell damage. The method is closely related to chromophore-assisted laser inactivation and photodynamic therapy, but is driven solely by light absorption, without the need for photochemical intermediates (particularly singlet oxygen). The mechanism of light-particle interaction was investigated by nanosecond time-resolved microscopy and by thermal modeling. The extent of light-induced damage was investigated by cell lethality, by cell membrane permeability, and by protein inactivation. Strong particle size dependence was found for these interactions. A technique based on light to target endogenous particles is already being exploited to treat pigmented cells in dermatology and ophthalmology. With exogenous particles, phamacokinetics and biodistribution studies are needed before the method can be evaluated against photodynamic therapy for cancer treatment. However, particles are unique, unlike photosensitizers, in that they can remain stable and inert in cells for extended periods. Thus they may be particularly useful for prelabeling cells in engineered tissue before implantation. Subsequent irradiation with laser pulses will allow control of the implanted cells (inactivation or modulation) in a noninvasive manner.
Figures

Similar articles
-
Thermal scalpel to target cancer.Expert Rev Med Devices. 2007 Mar;4(2):131-6. doi: 10.1586/17434440.4.2.131. Expert Rev Med Devices. 2007. PMID: 17359220 Review.
-
Gold nanoparticles propulsion from surface fueled by absorption of femtosecond laser pulse at their surface plasmon resonance.J Am Chem Soc. 2006 Oct 18;128(41):13330-1. doi: 10.1021/ja064328p. J Am Chem Soc. 2006. PMID: 17031925
-
Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water.Lasers Surg Med. 1996;19(1):23-31. doi: 10.1002/(SICI)1096-9101(1996)19:1<23::AID-LSM4>3.0.CO;2-S. Lasers Surg Med. 1996. PMID: 8836993
-
Controlled ablation of microtubules using a picosecond laser.Biophys J. 2004 Dec;87(6):4203-12. doi: 10.1529/biophysj.104.049528. Epub 2004 Sep 28. Biophys J. 2004. PMID: 15454403 Free PMC article.
-
Laser-based gene transfection and gene therapy.IEEE Trans Nanobioscience. 2008 Jun;7(2):111-9. doi: 10.1109/TNB.2008.2000742. IEEE Trans Nanobioscience. 2008. PMID: 18556259 Review.
Cited by
-
Cell-specific transmembrane injection of molecular cargo with gold nanoparticle-generated transient plasmonic nanobubbles.Biomaterials. 2012 Jul;33(21):5441-50. doi: 10.1016/j.biomaterials.2012.03.077. Epub 2012 Apr 20. Biomaterials. 2012. PMID: 22521612 Free PMC article.
-
Plasmonic photothermal therapy (PPTT) using gold nanoparticles.Lasers Med Sci. 2008 Jul;23(3):217-28. doi: 10.1007/s10103-007-0470-x. Epub 2007 Aug 3. Lasers Med Sci. 2008. PMID: 17674122 Review.
-
Elevation of plasma membrane permeability upon laser irradiation of extracellular microbubbles.Lasers Med Sci. 2010 Jul;25(4):587-94. doi: 10.1007/s10103-010-0773-1. Epub 2010 Mar 20. Lasers Med Sci. 2010. PMID: 20306103
-
Vapor bubble generation around gold nano-particles and its application to damaging of cells.Biomed Opt Express. 2011 Jan 11;2(2):291-304. doi: 10.1364/BOE.2.000291. Biomed Opt Express. 2011. PMID: 21339875 Free PMC article.
-
Near-Infrared-Responsive Cancer Photothermal and Photodynamic Therapy Using Gold Nanoparticles.Polymers (Basel). 2018 Aug 30;10(9):961. doi: 10.3390/polym10090961. Polymers (Basel). 2018. PMID: 30960886 Free PMC article. Review.
References
-
- Anderson, R. R. 2000. Lasers in dermatology—a critical update. J. Dermatol. 27:700–705. - PubMed
-
- Anderson, R. R., R. J. Margolis, S. Watenabe, T. Flotte, G. J. Hruza, and J. S. Dover. 1989. Selective photothermolysis of cutaneous pigmentation by Q-switched Nd:YAG laser pulses at 1064, 532, and 355 nm. J. Invest. Dermatol. 93:28–32. - PubMed
-
- Anderson, R. R., and J. A. Parrish. 1983. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 220:524–527. - PubMed
-
- Beck, S., T. Sakurai, B. K. Eustace, G. Beste, R. Schier, F. Rudert, and D. G. Jay. 2002. Fluorophore-assisted light inactivation: a high throughput tool for direct target validation of proteins. Proteomics. 2:247–255. - PubMed
-
- Brinkmann, R., G. Huettmann, J. Roegener, J. Roider, R. Birngruber, and C. P. Lin. 2000. Origin of retinal pigment epithelium cell damage by pulsed laser irradiance in the nanosecond to microsecond time regimen. Lasers Surg. Med. 27:451–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

