Thymidine phosphorylase induces angiogenesis in vivo and in vitro: an evaluation of possible mechanisms
- PMID: 12770927
- PMCID: PMC1573835
- DOI: 10.1038/sj.bjp.0705216
Thymidine phosphorylase induces angiogenesis in vivo and in vitro: an evaluation of possible mechanisms
Abstract
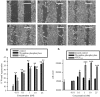
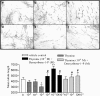
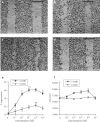
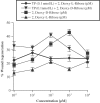
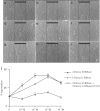
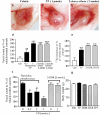
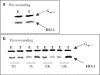
1 Thymidine phosphorylase (TP) is elevated in the plasma of cancer patients, and has been implicated in pathophysiological angiogenesis. However, the downstream signals underlying this implication remain obscure. The purpose of the present study was to examine the effects of TP on the neovascularisation response in vitro and in vivo. 2 Both TP and its catalytic product, 2-deoxy-D-ribose-1-phosphate, and downstream 2-deoxy-D-ribose (2-DDR) promoted endothelial tubulogenesis in vitro, and the regeneration of a wounded monolayer of endothelial cells without exerting any mitogenic effect. In vivo, both TP and 2-DDR promoted the development of functional vasculature into an avascular sponge. A TP inhibitor, 6-amino-5-chlorouracil, was able to partially reverse the effects of TP, but had no effect on the 2-DDR-induced angiogenesis. 3 Enhanced monolayer regeneration was observed with TP-cDNA-transfected bladder carcinoma cells. The transfection of TP-cDNA, however, did not confer any proliferative advantage. The regeneration of TP overexpressing cells was associated with a time-dependent expression of the enzyme haeme-oxygenase (HO-1). 4 The present study demonstrates that both TP and its ribose-sugar metabolites induce angiogenesis by mediating a cohesive interplay between carcinoma and endothelial cells. The induction of HO-1 in TP-transfected cells suggests that it could be a possible downstream signal for the angiogenic effects of TP. Furthermore, reducing sugars have been shown to induce oxidative stress, and ribose could be a possible cause for the upregulation of HO-1, which has been implicated in the release of angiogenic factors. Therefore, we postulate that 2-DDR could be mediating the angiogenic effects of TP possibly through an oxidative stress mechanism and additionally getting integrated in the endothelial metabolic machinery.
Figures
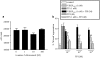

Similar articles
-
Suppression of thymidine phosphorylase-mediated angiogenesis and tumor growth by 2-deoxy-L-ribose.Cancer Res. 2002 May 15;62(10):2834-9. Cancer Res. 2002. PMID: 12019161
-
Prevention of hypoxia-induced apoptosis by the angiogenic factor thymidine phosphorylase.Biochem Biophys Res Commun. 1998 Dec 30;253(3):797-803. doi: 10.1006/bbrc.1998.9852. Biochem Biophys Res Commun. 1998. PMID: 9918807
-
The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression.Cancer Sci. 2004 Nov;95(11):851-7. doi: 10.1111/j.1349-7006.2004.tb02193.x. Cancer Sci. 2004. PMID: 15546501 Free PMC article. Review.
-
Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors.Cancer Res. 2000 Nov 15;60(22):6298-302. Cancer Res. 2000. PMID: 11103787
-
Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis.Biochem J. 1998 Aug 15;334 ( Pt 1)(Pt 1):1-8. doi: 10.1042/bj3340001. Biochem J. 1998. PMID: 9693094 Free PMC article. Review.
Cited by
-
Angiogenesis in abnormal uterine bleeding: a narrative review.Hum Reprod Update. 2023 Jul 5;29(4):457-485. doi: 10.1093/humupd/dmad004. Hum Reprod Update. 2023. PMID: 36857162 Free PMC article. Review.
-
Angiogenic biomarkers in children with congenital heart disease: possible implications.Ital J Pediatr. 2010 Apr 20;36:32. doi: 10.1186/1824-7288-36-32. Ital J Pediatr. 2010. PMID: 20406482 Free PMC article.
-
Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy.Curr Cancer Drug Targets. 2005 Dec;5(8):579-94. doi: 10.2174/156800905774932824. Curr Cancer Drug Targets. 2005. PMID: 16375664 Free PMC article. Review.
-
Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis.Circulation. 2008 Jan 15;117(2):231-41. doi: 10.1161/CIRCULATIONAHA.107.698316. Circulation. 2008. PMID: 18195184 Free PMC article. Review.
-
Paracrine stimulation of endothelial cell motility and angiogenesis by platelet-derived deoxyribose-1-phosphate.Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2631-8. doi: 10.1161/ATVBAHA.110.215855. Epub 2010 Sep 30. Arterioscler Thromb Vasc Biol. 2010. PMID: 20884872 Free PMC article.
References
-
- BOYLE J.J., WILSON B., BICKNELL R., HARROWER S., WEISSBERG P.L., FAN T.P. Expression of angiogenic factor thymidine phosphorylase and angiogenesis in human atherosclerosis. J. Pathol. 2000;192:234–242. - PubMed
-
- BROWN N.S., JONES A., FUJIYAMA C., HARRIS A.L., BICKNELL R. Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Cancer Res. 2000;60:6298–6302. - PubMed
-
- CHOI A.M., ALAM J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996;15:9–19. - PubMed
-
- CURRIE M.J., GUNNINGHAM S.P., HAN C., SCOTT P.A., ROBINSON B.A., HARRIS A.L., FOX S.B. Angiopoietin-1 is inversely related to thymidine phosphorylase expression in human breast cancer, indicating a role in vascular remodeling. Clin. Cancer Res. 2001;7:918–927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

