Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum
- PMID: 12770934
- PMCID: PMC1573843
- DOI: 10.1038/sj.bjp.0705243
Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum
Abstract
1 The polysaccharide from Ganoderma lucidum (PS-G) has been reported to enhance immune responses and to elicit antitumor effects. In our previous study, we found that PS-G efficiently inhibited spontaneously and Fas-enhanced neutrophil apoptosis when cultured in vitro. Since phagocytosis and chemotaxis play essential roles in host defense mediated by neutrophils, it is of great interest to know the effect of PS-G on these two cell functions, and the molecular events leading to these actions. 2 Using latex beads and heat-inactive Escherichia coli serving as particles for neutrophil engulfment, we found that PS-G is able to enhance phagocytic activity of human primary neutrophils and neutrophilic-phenotype cells differentiated from all trans retinoic acid-treated HL-60 cells. 3 Chemotactic assay using Boyden chamber also revealed the ability of PS-G to increase neutrophil migration. 4 Exposure of neutrophils to PS-G time dependently caused increases in protein kinase C (PKC), p38 mitogen-activated protein kinase (MAPK), Hck, and Lyn activities. 5 Results with specific kinase inhibitors indicate that phagocytic action of PS-G was reduced by the presence of wortmannin (Phosphatidylinositol 3-kinase, PI3K inhibitor), pyrazolpyrimidine 2 (Src-family tyrosine kinase inhibitor), Ro318220 (PKC inhibitor), and SB203580 (p38 MAPK inhibitor), but not by PD98059 (mitogen-activated protein/ERK kinase inhibitor). Moreover, chemotactic action of PS-G requires the activities of PI3K, p38 MAPK, Src tyrosine kinases and PKC. 6 All these results demonstrate the abilities of PS-G to enhance neutrophil function in phagocytosis and chemotaxis, and further provide evidence to strengthen the beneficial remedy of G. lucidum in human to enhance defense system.
Figures

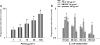


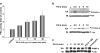

Similar articles
-
Polysaccharide purified from Ganoderma lucidum inhibits spontaneous and Fas-mediated apoptosis in human neutrophils through activation of the phosphatidylinositol 3 kinase/Akt signaling pathway.J Leukoc Biol. 2002 Jul;72(1):207-16. J Leukoc Biol. 2002. PMID: 12101282
-
Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases.Ann N Y Acad Sci. 2006 Jun;1069:463-71. doi: 10.1196/annals.1351.045. Ann N Y Acad Sci. 2006. PMID: 16855174
-
Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways: role in activation of reduced nicotinamide adenine dinucleotide oxidase.J Immunol. 1997 Nov 15;159(10):5070-8. J Immunol. 1997. PMID: 9366435
-
Roles of PI3K in neutrophil function.Curr Top Microbiol Immunol. 2004;282:165-75. doi: 10.1007/978-3-642-18805-3_6. Curr Top Microbiol Immunol. 2004. PMID: 14594217 Review.
-
Organization and functions of glycolipid-enriched microdomains in phagocytes.Biochim Biophys Acta. 2015 Jan;1851(1):90-7. doi: 10.1016/j.bbalip.2014.06.009. Epub 2014 Jun 23. Biochim Biophys Acta. 2015. PMID: 24968752 Review.
Cited by
-
The multifaceted effects of polysaccharides isolated from Dendrobium huoshanense on immune functions with the induction of interleukin-1 receptor antagonist (IL-1ra) in monocytes.PLoS One. 2014 Apr 4;9(4):e94040. doi: 10.1371/journal.pone.0094040. eCollection 2014. PLoS One. 2014. PMID: 24705413 Free PMC article.
-
Ganoderma lucidum spore powder alleviates rheumatoid arthritis-associated pain hypersensitivity through inhibiting accumulation, N1 polarization, and ROS production of neutrophils in mice.Front Immunol. 2025 Apr 30;16:1569295. doi: 10.3389/fimmu.2025.1569295. eCollection 2025. Front Immunol. 2025. PMID: 40370462 Free PMC article.
-
Matrin3 (MATR3) Expression Is Associated with Hemophagocytosis.Biomedicines. 2022 Sep 1;10(9):2161. doi: 10.3390/biomedicines10092161. Biomedicines. 2022. PMID: 36140262 Free PMC article.
-
In Vitro Effects of St. John's Wort Extract Against Inflammatory and Oxidative Stress and in the Phagocytic and Migratory Activity of Mouse SIM-A9 Microglia.Front Pharmacol. 2020 Dec 3;11:603575. doi: 10.3389/fphar.2020.603575. eCollection 2020. Front Pharmacol. 2020. PMID: 33628177 Free PMC article.
-
Polysaccharides with Arabinose: Key Players in Reducing Chronic Inflammation and Enhancing Immune Health in Aging.Molecules. 2025 Mar 6;30(5):1178. doi: 10.3390/molecules30051178. Molecules. 2025. PMID: 40076400 Free PMC article. Review.
References
-
- BHARADWAJ D., MOLD C., MARKHAM E., DU CLOS T.W. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J. Immunol. 2001;166:6735–6741. - PubMed
-
- BOBER L.A., GRACE M.J., PUGLIESE-SIVO C., ROJAS-TRIANA A., WATERS T., SULLIVAN L.M., NARULA S.K. The effect of GM-CSF and G-CSF on human neutrophil function. Immunopharmacology. 1995;29:111–119. - PubMed
-
- BOHN J.A., BEMILLER J.N. (1→3)-D-glucans as biological response modifiers: a review of structure–functional activity relationships. Carbohydrate Polym. 1995;28:3–14.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

