Keratin 8 protection of placental barrier function
- PMID: 12771125
- PMCID: PMC2199358
- DOI: 10.1083/jcb.200210004
Keratin 8 protection of placental barrier function
Abstract
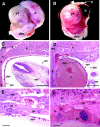
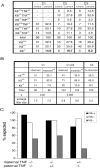
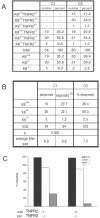
The intermediate filament protein keratin 8 (K8) is critical for the development of most mouse embryos beyond midgestation. We find that 68% of K8-/- embryos, in a sensitive genetic background, are rescued from placental bleeding and subsequent death by cellular complementation with wild-type tetraploid extraembryonic cells. This indicates that the primary defect responsible for K8-/- lethality is trophoblast giant cell layer failure. Furthermore, the genetic absence of maternal but not paternal TNF doubles the number of viable K8-/- embryos. Finally, we show that K8-/- concepti are more sensitive to a TNF-dependent epithelial apoptosis induced by the administration of concanavalin A (ConA) to pregnant mothers. The ConA-induced failure of the trophoblast giant cell barrier results in hematoma formation between the trophoblast giant cell layer and the embryonic yolk sac in a phenocopy of dying K8-deficient concepti in a sensitive genetic background. We conclude the lethality of K8-/- embryos is due to a TNF-sensitive failure of trophoblast giant cell barrier function. The keratin-dependent protection of trophoblast giant cells from a maternal TNF-dependent apoptotic challenge may be a key function of simple epithelial keratins.
Figures

Similar articles
-
Targeted deletion of keratins 18 and 19 leads to trophoblast fragility and early embryonic lethality.EMBO J. 2000 Oct 2;19(19):5060-70. doi: 10.1093/emboj/19.19.5060. EMBO J. 2000. PMID: 11013209 Free PMC article.
-
Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis.J Cell Biol. 2000 Apr 3;149(1):17-22. doi: 10.1083/jcb.149.1.17. J Cell Biol. 2000. PMID: 10747083 Free PMC article.
-
Rescue of keratin 18/19 doubly deficient mice using aggregation with tetraploid embryos.Eur J Cell Biol. 2005 Mar;84(2-3):355-61. doi: 10.1016/j.ejcb.2004.12.014. Eur J Cell Biol. 2005. PMID: 15819413
-
Keratin modifications and solubility properties in epithelial cells and in vitro.Subcell Biochem. 1998;31:105-40. Subcell Biochem. 1998. PMID: 9932491 Review.
-
Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease.Biochem Cell Biol. 2001;79(5):543-55. Biochem Cell Biol. 2001. PMID: 11716296 Review.
Cited by
-
Keratins modulate colonocyte electrolyte transport via protein mistargeting.J Cell Biol. 2004 Mar 15;164(6):911-21. doi: 10.1083/jcb.200308103. Epub 2004 Mar 8. J Cell Biol. 2004. PMID: 15007064 Free PMC article.
-
Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity.Tissue Barriers. 2016 May 2;4(3):e1178368. doi: 10.1080/21688370.2016.1178368. eCollection 2016 Jul-Sep. Tissue Barriers. 2016. PMID: 27583190 Free PMC article. Review.
-
Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion.Genes Dev. 2006 May 15;20(10):1353-64. doi: 10.1101/gad.1387406. Genes Dev. 2006. PMID: 16702408 Free PMC article.
-
Expression of Keratin 8 and TNF-Related Apoptosis-I Inducing Ligand (TRAIL) in Down Syndrome Placentas.Placenta. 2008 Apr;29(4):382-4. doi: 10.1016/j.placenta.2008.01.013. Epub 2008 Mar 17. Placenta. 2008. PMID: 18343496 Free PMC article. No abstract available.
-
Intermediate filaments: a historical perspective.Exp Cell Res. 2007 Jun 10;313(10):1981-94. doi: 10.1016/j.yexcr.2007.04.007. Epub 2007 Apr 11. Exp Cell Res. 2007. PMID: 17493611 Free PMC article. Review.
References
-
- Baribault, H., J. Price, K. Miyai, and R.G. Oshima. 1993. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 7:1191–1202. - PubMed
-
- Baribault, H., J. Penner, R.V. Iozzo, and M. Wilson-Heiner. 1994. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 8:2964–2973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

