Custom distinctions in the interaction of G-protein beta subunits with N-type (CaV2.2) versus P/Q-type (CaV2.1) calcium channels
- PMID: 12771191
- PMCID: PMC2217353
- DOI: 10.1085/jgp.200208770
Custom distinctions in the interaction of G-protein beta subunits with N-type (CaV2.2) versus P/Q-type (CaV2.1) calcium channels
Abstract
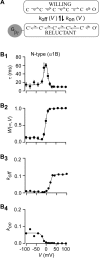
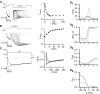
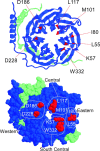
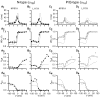
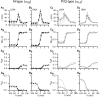
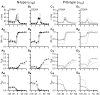
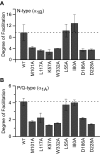
Inhibition of N- (Cav2.2) and P/Q-type (Cav2.1) calcium channels by G-proteins contribute importantly to presynaptic inhibition as well as to the effects of opiates and cannabinoids. Accordingly, elucidating the molecular mechanisms underlying G-protein inhibition of voltage-gated calcium channels has been a major research focus. So far, inhibition is thought to result from the interaction of multiple proposed sites with the Gbetagamma complex (Gbetagamma). Far less is known about the important interaction sites on Gbetagamma itself. Here, we developed a novel electrophysiological paradigm, "compound-state willing-reluctant analysis," to describe Gbetagamma interaction with N- and P/Q-type channels, and to provide a sensitive and efficient screen for changes in modulatory behavior over a broad range of potentials. The analysis confirmed that the apparent (un)binding kinetics of Gbetagamma with N-type are twofold slower than with P/Q-type at the voltage extremes, and emphasized that the kinetic discrepancy increases up to ten-fold in the mid-voltage range. To further investigate apparent differences in modulatory behavior, we screened both channels for the effects of single point alanine mutations within four regions of Gbeta1, at residues known to interact with Galpha. These residues might thereby be expected to interact with channel effectors. Of eight mutations studied, six affected G-protein modulation of both N- and P/Q-type channels to varying degrees, and one had no appreciable effect on either channel. The remaining mutation was remarkable for selective attenuation of effects on P/Q-, but not N-type channels. Surprisingly, this mutation decreased the (un)binding rates without affecting its overall affinity. The latter mutation suggests that the binding surface on Gbetagamma for N- and P/Q-type channels are different. Also, the manner in which this last mutation affected P/Q-type channels suggests that some residues may be important for "steering" or guiding the protein into the binding pocket, whereas others are important for simply binding to the channel.
Figures



References
-
- Albsoul-Younes, A.M., P.M. Sternweis, P. Zhao, H. Nakata, S. Nakajima, Y. Nakajima, and T. Kozasa. 2001. Interaction sites of the G protein β subunit with brain G protein-coupled inward rectifier K+ channel. J. Biol. Chem. 276:12712–12717. - PubMed
-
- Artim, D.E., and S.D. Meriney. 2000. G-protein-modulated Ca(2+) current with slowed activation does not alter the kinetics of action potential-evoked Ca(2+) current. J. Neurophysiol. 84:2417–2425. - PubMed
-
- Atkins, P. 1998. Chapter 27: Molecular reaction dynamics. Physical Chemistry. W.H. Freeman and Company, New York. 830–843.

