Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement
- PMID: 12771195
- PMCID: PMC2217352
- DOI: 10.1085/jgp.200308813
Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement
Abstract
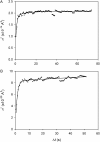
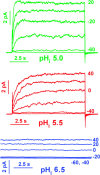
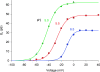
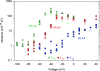
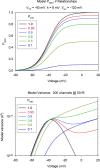
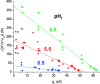
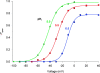
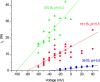
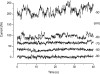
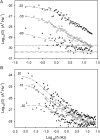
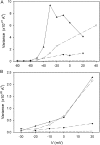
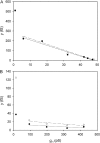
Voltage-gated proton channels were studied under voltage clamp in excised, inside-out patches of human eosinophils, at various pHi with pHo 7.5 or 6.5 pipette solutions. H+ current fluctuations were observed consistently when the membrane was depolarized to voltages that activated H+ current. At pHi < or = 5.5 the variance increased nonmonotonically with depolarization to a maximum near the midpoint of the H+ conductance-voltage relationship, gH-V, and then decreased, supporting the idea that the noise is generated by H+ channel gating. Power spectral analysis indicated Lorentzian and 1/f components, both related to H+ currents. Unitary H+ current amplitude was estimated from stationary or quasi-stationary variance, sigmaH2. We analyze sigmaH2 data obtained at various voltages on a linearized plot that provides estimates of both unitary conductance and the number of channels in the patch, without requiring knowledge of open probability. The unitary conductance averaged 38 fS at pHi 6.5, and increased nearly fourfold to 140 fS at pHi 5.5, but was independent of pHo. In contrast, the macroscopic gH was only 1.8-fold larger at pHi 5.5 than at pHi 6.5. The maximum H+ channel open probability during large depolarizations was 0.75 at pHi 6.5 and 0.95 at pHi 5.5. Because the unitary conductance increases at lower pHi more than the macroscopic gH, the number of functional channels must decrease. Single H+ channel currents were too small to record directly at physiological pH, but at pHi < or = 5.5 near Vthreshold (the voltage at which gH turns on), single channel-like current events were observed with amplitudes 7-16 fA.
Figures
Similar articles
-
Voltage-activated proton currents in membrane patches of rat alveolar epithelial cells.J Physiol. 1995 Dec 1;489 ( Pt 2)(Pt 2):299-307. doi: 10.1113/jphysiol.1995.sp021051. J Physiol. 1995. PMID: 8847626 Free PMC article.
-
The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient.J Gen Physiol. 1995 Jun;105(6):861-96. doi: 10.1085/jgp.105.6.861. J Gen Physiol. 1995. PMID: 7561747 Free PMC article.
-
Interactions between NADPH oxidase-related proton and electron currents in human eosinophils.J Physiol. 2001 Sep 15;535(Pt 3):767-81. doi: 10.1111/j.1469-7793.2001.00767.x. J Physiol. 2001. PMID: 11559774 Free PMC article.
-
Pharmacology of voltage-gated proton channels.Curr Pharm Des. 2007;13(23):2400-20. doi: 10.2174/138161207781368675. Curr Pharm Des. 2007. PMID: 17692009 Review.
-
Diversity of voltage gated proton channels.Front Biosci. 2003 Sep 1;8:s1266-79. doi: 10.2741/1191. Front Biosci. 2003. PMID: 12957841 Review.
Cited by
-
Temperature Dependent Activity of the Voltage-Gated Proton Channel.Adv Exp Med Biol. 2024;1461:109-125. doi: 10.1007/978-981-97-4584-5_8. Adv Exp Med Biol. 2024. PMID: 39289277 Review.
-
Concerted action of two cation filters in the aquaporin water channel.EMBO J. 2009 Aug 5;28(15):2188-94. doi: 10.1038/emboj.2009.182. Epub 2009 Jul 2. EMBO J. 2009. PMID: 19574955 Free PMC article.
-
Molecular and functional characterization of the voltage-gated proton channel in zebrafish neutrophils.Physiol Rep. 2017 Aug;5(15):e13345. doi: 10.14814/phy2.13345. Physiol Rep. 2017. PMID: 28774948 Free PMC article.
-
Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family.Physiol Rev. 2013 Apr;93(2):599-652. doi: 10.1152/physrev.00011.2012. Physiol Rev. 2013. PMID: 23589829 Free PMC article. Review.
-
Characterization of a highly efficient blue-shifted channelrhodopsin from the marine alga Platymonas subcordiformis.J Biol Chem. 2013 Oct 11;288(41):29911-22. doi: 10.1074/jbc.M113.505495. Epub 2013 Aug 30. J Biol Chem. 2013. PMID: 23995841 Free PMC article.
References
-
- Bezrukov, S.M., and M. Winterhalter. 2000. Examining noise sources at the single-molecule level: 1/f noise of an open maltoporin channel. Phys. Rev. Lett. 85:202–205. - PubMed

