The Drosophila Corto protein interacts with Polycomb-group proteins and the GAGA factor
- PMID: 12771214
- PMCID: PMC156716
- DOI: 10.1093/nar/gkg381
The Drosophila Corto protein interacts with Polycomb-group proteins and the GAGA factor
Abstract
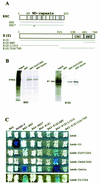
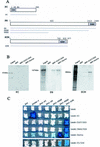
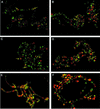
In Drosophila, PcG complexes provide heritable transcriptional silencing of target genes. Among them, the ESC/E(Z) complex is thought to play a role in the initiation of silencing whereas other complexes such as the PRC1 complex are thought to maintain it. PcG complexes are thought to be recruited to DNA through interaction with DNA binding proteins such as the GAGA factor, but no direct interactions between the constituents of PcG complexes and the GAGA factor have been reported so far. The Drosophila corto gene interacts with E(z) as well as with genes encoding members of maintenance complexes, suggesting that it could play a role in the transition between the initiation and maintenance of PcG silencing. Moreover, corto also interacts genetically with Trl, which encodes the GAGA factor, suggesting that it may serve as a mediator in recruiting PcG complexes. Here, we show that Corto bears a chromo domain and we provide evidence for in vivo association of Corto with ESC and with PC in embryos. Moreover, we show by GST pull-down and two-hybrid experiments that Corto binds to E(Z), ESC, PH, SCM and GAGA and co-localizes with these proteins on a few sites on polytene chromosomes. These results reinforce the idea that Corto plays a role in PcG silencing, perhaps by confering target specificity.
Figures




Similar articles
-
Corto and DSP1 interact and bind to a maintenance element of the Scr Hox gene: understanding the role of Enhancers of trithorax and Polycomb.BMC Biol. 2006 Apr 14;4:9. doi: 10.1186/1741-7007-4-9. BMC Biol. 2006. PMID: 16613610 Free PMC article.
-
A 1-megadalton ESC/E(Z) complex from Drosophila that contains polycomblike and RPD3.Mol Cell Biol. 2003 May;23(9):3352-62. doi: 10.1128/MCB.23.9.3352-3362.2003. Mol Cell Biol. 2003. PMID: 12697833 Free PMC article.
-
The Drosophila pho-like gene encodes a YY1-related DNA binding protein that is redundant with pleiohomeotic in homeotic gene silencing.Development. 2003 Jan;130(2):285-94. doi: 10.1242/dev.00204. Development. 2003. PMID: 12466196
-
The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3.Curr Opin Genet Dev. 2004 Apr;14(2):155-64. doi: 10.1016/j.gde.2004.02.001. Curr Opin Genet Dev. 2004. PMID: 15196462 Review.
-
Heritable chromatin states induced by the Polycomb and trithorax group genes.Novartis Found Symp. 1998;214:51-61; discussion 61-6, 104-13. doi: 10.1002/9780470515501.ch4. Novartis Found Symp. 1998. PMID: 9601011 Review.
Cited by
-
Investigating the genetic circuitry of mastermind in Drosophila, a notch signal effector.Genetics. 2007 Dec;177(4):2493-505. doi: 10.1534/genetics.107.080994. Genetics. 2007. PMID: 18073442 Free PMC article.
-
The Arabidopsis GAGA-Binding Factor BASIC PENTACYSTEINE6 Recruits the POLYCOMB-REPRESSIVE COMPLEX1 Component LIKE HETEROCHROMATIN PROTEIN1 to GAGA DNA Motifs.Plant Physiol. 2015 Jul;168(3):1013-24. doi: 10.1104/pp.15.00409. Epub 2015 May 29. Plant Physiol. 2015. PMID: 26025051 Free PMC article.
-
Functional conservation of Asxl2, a murine homolog for the Drosophila enhancer of trithorax and polycomb group gene Asx.PLoS One. 2009;4(3):e4750. doi: 10.1371/journal.pone.0004750. Epub 2009 Mar 9. PLoS One. 2009. PMID: 19270745 Free PMC article.
-
Piwi maintains germline stem cells and oogenesis in Drosophila through negative regulation of Polycomb group proteins.Nat Genet. 2016 Mar;48(3):283-91. doi: 10.1038/ng.3486. Epub 2016 Jan 18. Nat Genet. 2016. PMID: 26780607 Free PMC article.
-
New partners in regulation of gene expression: the enhancer of Trithorax and Polycomb Corto interacts with methylated ribosomal protein l12 via its chromodomain.PLoS Genet. 2012;8(10):e1003006. doi: 10.1371/journal.pgen.1003006. Epub 2012 Oct 11. PLoS Genet. 2012. PMID: 23071455 Free PMC article.
References
-
- Lewis E.B. (1978) A gene complex controlling segmentation in Drosophila. Nature, 276, 565–570. - PubMed
-
- McKeon J., Slade,E., Sinclair,D.A., Cheng,N., Couling,M. and Brock,H.W. (1994) Mutations in some Polycomb group genes of Drosophila interfere with regulation of segmentation genes. Mol. Gen. Genet., 244, 474–483. - PubMed
-
- Daubresse G., Deuring,R., Moore,L., Papoulas,O., Zakrajsek,I., Waldrip,W.R., Scott,M.P., Kennison,J.A. and Tamkun,J.W. (1999) The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development, 126, 1175–1187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

