Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients
- PMID: 12771924
- PMCID: PMC2377109
- DOI: 10.1038/sj.bjc.6600918
Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients
Abstract
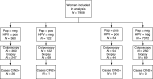
In a prospective cohort study 8466 women attending routine cervical cancer screening were recruited. Colposcopy was performed on women with any degree of atypia on cytology and/or a positive high-risk human papillomavirus (HPV)-DNA test (HC2; Hybrid Capture 2((c))), and for a randomly selected sample of 3.4% women with negative findings on both. Quality control included reviews of cytology, histology, colposcopy images and retesting of samples with polymerase chain reaction. Test diagnostic performances were based on 7908 women who had complete baseline and follow-up results. Routine histology identified 86 women with high-grade cervical intraepithelial neoplasia (CIN2+), which was confirmed by review histology in only 46 cases. Sensitivity of routine cytology for the detection of CIN2+ was 43.5%, with a specificity, positive predictive value (PPV), negative predictive value (NPV) of 98.0, 11.4 and 99.7%, respectively. Sensitivity of the HC2 test for the detection of CIN2+ was 97.8%, with a specificity, PPV and NPV, of 95.3, 10.9 and 100%, respectively. No high-grade neoplasia was detected in the randomly selected control group. A negative HPV-test result, even in combination with a positive Papanicolaou (Pap) result, virtually excluded any risk of underlying high-grade disease, but this was not the case for a negative Pap result. These data show that HPV testing is of value for the detection or exclusion of prevalent CIN in a routine cervical cancer-screening setting and could be used for further risk classification of women for follow-up management.
Figures
References
-
- Bosch XF, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV (1995) Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst 87: 796–802 - PubMed
-
- Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G (1995) Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis 171: 1026–1030 - PubMed
-
- Ferlay J, Parkin DM, Pisani Pe (1988) EUCAN Database: Cancer Incidence and Mortality in Europe. International Agency for Research on Cancer–World Health Organisation. IARC Press: Lyon