Apoptosis induction in renal cell carcinoma by TRAIL and gamma-radiation is impaired by deficient caspase-9 cleavage
- PMID: 12771998
- PMCID: PMC2377130
- DOI: 10.1038/sj.bjc.6600984
Apoptosis induction in renal cell carcinoma by TRAIL and gamma-radiation is impaired by deficient caspase-9 cleavage
Abstract
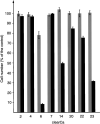
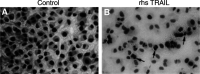
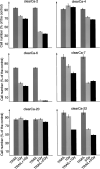
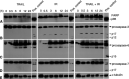
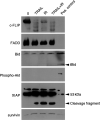
TNF-related apoptosis-inducing ligand (TRAIL APO-2L) is a member of the TNF family and induces apoptosis in cancer cells without affecting most non-neoplastic cells. The present investigation is focused on apoptosis induction by combined exposure to TRAIL and ionising radiation (IR) in human renal cell carcinoma (RCC) cell lines. Here, we demonstrate that all RCC cell lines coexpress TRAIL and the death-inducing receptors, TRAIL-R1 and TRAIL-R2. Exposure to TRAIL alone induced marked apoptosis in three out of eight RCC cell lines. Combined exposure to TRAIL and IR resulted in a sensitisation to TRAIL-induced apoptosis in one RCC cell line only. Enhanced apoptosis induction by TRAIL in combination with IR was paralleled by an increase in PARP cleavage and activation of executioner caspase-3, whereas caspases-6 and -7 were not involved. Moreover, exposure to TRAIL and/or IR resulted in a marked activation of initiator caspase-8, possibly augmented by the observed reduction of inhibitory c-FLIP expression. In contrast to other tumour types, activation of initiator caspase-9 was not detectable in our RCC model system after exposure to TRAIL and/or IR. This lack of caspase-9 activation might be related to an impaired 'crosstalk' with the caspase-8 pathway as suggested by the missing Bid cleavage and to the appearance of an XIAP cleavage product known to inhibit caspase-9 activation. Deficient activation of caspase-9, therefore, might contribute to the clinically known resistance of human RCC against IR and also argues against an effective combination therapy with TRAIL and IR in this tumour type.
Figures


References
-
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104: 155–162 - PMC - PubMed
-
- Belka C, Rudner J, Wesselborg S, Stepczynska A, Marini P, Lepple-Wienhues A, Faltin H, Bamberg M, Budach W, Schulze-Osthoff K (2000) Differential role of caspase-8 and BID activation during radiation- and CD95-induced apoptosis. Oncogene 19: 1181–1190 - PubMed
-
- Belka C, Schmid B, Marini P, Durand E, Rudner J, Faltin H, Bamberg M, Schulze-Osthoff K, Budach W (2001) Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene 20: 2190–2196 - PubMed
-
- Brown JM, Wouters BG (1999) Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 59: 1391–1399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

