Development, validity and responsiveness of the Clinical COPD Questionnaire
- PMID: 12773199
- PMCID: PMC156640
- DOI: 10.1186/1477-7525-1-13
Development, validity and responsiveness of the Clinical COPD Questionnaire
Abstract
Background: The new Global Obstructive Lung Disease (GOLD) guidelines advice to focus treatment in Chronic Obstructive Pulmonary Disease (COPD) on improvement of functional state, prevention of disease progression and minimization of symptoms. So far no validated questionnaires are available to measure symptom and functional state in daily clinical practice. The aim of this study was to develop and validate the Clinical COPD Questionnaire (CCQ).
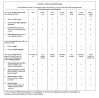
Methods: Qualitative research with patients and clinicians was performed to generate possible items to evaluate clinical COPD control. Thereafter, an item reduction questionnaire was sent to 77 international experts. Sixty-seven experts responded and the 10 most important items, divided into 3 domains (symptoms, functional and mental state) were included in the CCQ (scale: 0 = best, 6 = worst).
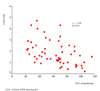
Results: Cross-sectional data were collected from 119 subjects (57 COPD, GOLD stage I-III; 18 GOLD stage 0 and 44 (ex)smokers). Cronbach's alpha was high (0.91). The CCQ scores in patients (GOLD 0-III) were significantly higher than in healthy (ex)smokers. Furthermore, significant correlations were found between the CCQ total score and domains of the SF-36 (rho = 0.48 to rho = 0.69) and the SGRQ (rho = 0.67 to rho = 0.72). In patients with COPD, the correlation between the CCQ and FEV1%pred was rho =-0.49. Test-retest reliability was determined in 20 subjects in a 2-week interval (Intra Class Coefficient = 0.94). Thirty-six smokers with and without COPD showed significant improvement in the CCQ after 2 months smoking cessation, indicating the responsiveness of the CCQ.
Conclusion: The CCQ is a self-administered questionnaire specially developed to measure clinical control in patients with COPD. Data support the validity, reliability and responsiveness of this short and easy to administer questionnaire.
Figures
References
-
- Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, Yernault JC, Decramer M, Higenbottam T, Postma DS, Rees J. Optimal assessment and management of chronic obstructive pulmonary disease (COPD): a consensus statement of the European Respiratory Society. Eur Respir J. 1995;8:1398–1420. doi: 10.1183/09031936.95.08081398. - DOI - PubMed
-
- Feenstra TL, van Genugten ML, Hoogenveen RT, Wouters EF, Rutten-van Molken MP. The impact of aging and smoking on the future of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164:590–596. - PubMed
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of chronic airflow limitation – the St George Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

