Molecular origins for the dominant negative function of human glucocorticoid receptor beta
- PMID: 12773573
- PMCID: PMC156139
- DOI: 10.1128/MCB.23.12.4319-4330.2003
Molecular origins for the dominant negative function of human glucocorticoid receptor beta
Abstract
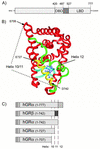
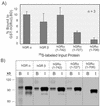
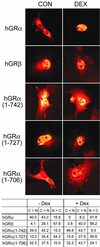
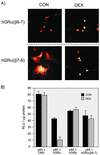
This study molecularly elucidates the basis for the dominant negative mechanism of the glucocorticoid receptor (GR) isoform hGRbeta, whose overexpression is associated with human glucocorticoid resistance. Using a series of truncated hGRalpha mutants and sequential mutagenesis to generate a series of hGRalpha/beta hybrids, we find that the absence of helix 12 is neither necessary nor sufficient for the GR dominant negative phenotype. Moreover, we have localized the dominant negative activity of hGRbeta to two residues and found that nuclear localization, in addition to heterodimerization, is a critical feature of the dominant negative activity. Molecular modeling of wild-type and mutant hGRalpha and hGRbeta provides structural insight and a potential physical explanation for the lack of hormone binding and the dominant negative actions of hGRbeta.
Figures





References
-
- Allgood, V. E., R. H. Oakley, and J. A. Cidlowski. 1993. Modulation by vitamin B6 of glucocorticoid receptor-mediated gene expression requires transcription factors in addition to the glucocorticoid receptor. J. Biol. Chem. 268:: 20870-20876. - PubMed
-
- Baldi, P., S. Brunak, P. Frasconi, G. Soda, and G. Pollastri. 1999. Exploiting the past and the future in protein secondary structure prediction. Bioinformatics 15:937-946. - PubMed
-
- Barroso, I., M. Gurnell, V. E. F. Crowley, M. Agostini, J. W. Schwabe, M. A. Soos, G. L. Maslen, T. D. M. Williams, H. Lewis, A. J. Schafer, V. K. K. Chatterjee, and S. O'Rahilly. 1999. Dominant negative mutations in human PPAR gamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402:880-883. - PubMed
-
- Bledsoe, R. K., V. G. Montana, T. B. Stanley, C. J. Delves, C. J. Apolito, D. D. McKee, T. G. Consler, D. J. Parks, E. L. Stewart, T. M. Willson, M. H. Lambert, J. T. Moore, K. H. Pearce, and H. E. Xu. 2002. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93-105. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
