Neural correlates of Pavlovian conditioning in components of the neural network supporting ciliary locomotion in Hermissenda
- PMID: 12773585
- PMCID: PMC202311
- DOI: 10.1101/lm.58603
Neural correlates of Pavlovian conditioning in components of the neural network supporting ciliary locomotion in Hermissenda
Abstract
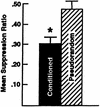
Pavlovian conditioning in Hermissenda consists of pairing light, the conditioned stimulus (CS) with activation of statocyst hair cells, the unconditioned stimulus (US). Conditioning produces CS-elicited foot shortening and inhibition of light-elicited locomotion, the two conditioned responses (CRs). Conditioning correlates have been identified in the primary sensory neurons (photoreceptors) of the CS pathway, interneurons that receive monosynaptic input from identified photoreceptors, and putative pedal motor neurons. While cellular mechanisms of acquisition produced by the synaptic interaction between the CS and US pathways are well-documented, little is known about the mechanisms responsible for the generation or expression of the CR. Here we show that in conditioned animals light reduced tonic firing of ciliary activating pedal neurons (VP1) below their pre-CS baseline levels. In contrast, pseudorandom controls expressed a significant increase in CS-elicited tonic firing of VP1 as compared to pre-CS baseline activity. Identified interneurons in the visual pathway that have established polysynaptic connections with VP1 were examined in conditioned animals and pseudorandom controls. Depolarization of identified type Ie interneurons with extrinsic current elicited a significant increase in IPSPs recorded in VP1 pedal neurons of conditioned animals as compared with pseudorandom controls. Conditioning also enhanced intrinsic excitability of type Ie interneurons of conditioned animals as compared to pseudorandom controls. Light evoked a modest increase in IPSP frequency in VP1 of conditioned preparations and a significant decrease in IPSP frequency in VP1 of pseudorandom controls. Our results show that a combination of synaptic facilitation and intrinsic enhanced excitability in identified components of the CS pathway may explain light-elicited inhibition of locomotion in conditioned animals.
Figures
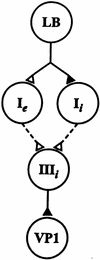




Similar articles
-
Interneuronal projections to identified cilia-activating pedal neurons in Hermissenda.J Neurophysiol. 2003 May;89(5):2420-9. doi: 10.1152/jn.01047.2002. J Neurophysiol. 2003. PMID: 12740402
-
Statocyst hair cell activation of identified interneurons and foot contraction motor neurons in Hermissenda.J Neurophysiol. 2004 Jun;91(6):2874-83. doi: 10.1152/jn.00028.2004. Epub 2004 Feb 25. J Neurophysiol. 2004. PMID: 14985407
-
Differential expression of correlates of classical conditioning in identified medial and lateral type A photoreceptors of Hermissenda.J Neurosci. 1993 Jul;13(7):2889-97. doi: 10.1523/JNEUROSCI.13-07-02889.1993. J Neurosci. 1993. PMID: 8331378 Free PMC article.
-
Pavlovian conditioning of Hermissenda: current cellular, molecular, and circuit perspectives.Learn Mem. 2004 May-Jun;11(3):229-38. doi: 10.1101/lm.70704. Learn Mem. 2004. PMID: 15169851 Review.
-
Pavlovian conditioning in Hermissenda: a circuit analysis.Biol Bull. 2006 Jun;210(3):289-97. doi: 10.2307/4134565. Biol Bull. 2006. PMID: 16801502 Review.
Cited by
-
Extending in vitro conditioning in Aplysia to analyze operant and classical processes in the same preparation.Learn Mem. 2004 Jul-Aug;11(4):412-20. doi: 10.1101/lm.74404. Epub 2004 Jul 14. Learn Mem. 2004. PMID: 15254218 Free PMC article.
-
Sensory regulation of network components underlying ciliary locomotion in Hermissenda.J Neurophysiol. 2008 Nov;100(5):2496-506. doi: 10.1152/jn.90759.2008. Epub 2008 Sep 3. J Neurophysiol. 2008. PMID: 18768639 Free PMC article.
-
5-HT and GABA modulate intrinsic excitability of type I interneurons in Hermissenda.J Neurophysiol. 2009 Nov;102(5):2825-33. doi: 10.1152/jn.00477.2009. Epub 2009 Aug 26. J Neurophysiol. 2009. PMID: 19710377 Free PMC article.
-
Polysensory interneuronal projections to foot contractile pedal neurons in Hermissenda.J Neurophysiol. 2009 Feb;101(2):824-33. doi: 10.1152/jn.91079.2008. Epub 2008 Dec 10. J Neurophysiol. 2009. PMID: 19073803 Free PMC article.
-
Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the reduction in A-type transient K+ current produced by one-trial in vitro conditioning of Hermissenda.J Neurosci. 2005 May 11;25(19):4793-800. doi: 10.1523/JNEUROSCI.5256-04.2005. J Neurosci. 2005. PMID: 15888654 Free PMC article.
References
-
- Akaike, T. and Alkon, D.L. 1980. Sensory convergence on central visual neurons in Hermissenda. J. Neurophysiol. 44: 501–513. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
