The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells
- PMID: 12773623
- PMCID: PMC165881
- DOI: 10.1073/pnas.1232342100
The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells
Abstract
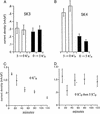
The question is, does the isoform hSK4, also designated KCNN4, represent the small conductance, Ca2+-activated K+ channel (Gardos channel) in human red blood cells? We have analyzed human reticulocyte RNA by RT-PCR, and, of the four isoforms of SK channels known, only SK4 was found. Northern blot analysis of purified and synchronously growing human erythroid progenitor cells, differentiating from erythroblasts to reticulocytes, again showed only the presence of SK4. Western blot analysis, with an anti-SK4 antibody, showed that human erythroid progenitor cells and, importantly, mature human red blood cell ghost membranes, both expressed the SK4 protein. The Gardos channel is known to turn on, given inside Ca2+, in the presence but not the absence of external Ko+ and remains refractory to Ko+ added after exposure to inside Ca2+. Heterologously expressed SK4, but not SK3, also shows this behavior. In inside-out patches of red cell membranes, the open probability (Po) of the Gardos channel is markedly reduced when the temperature is raised from 27 to 37 degrees C. Net K+ efflux of intact red cells is also reduced by increasing temperature, as are the Po values of inside-out patches of Chinese hamster ovary cells expressing SK4 (but not SK3). Thus the envelope of evidence indicates that SK4 is the gene that codes for the Gardos channel in human red blood cells. This channel is important pathophysiologically, because it represents the major pathway for cell shrinkage via KCl and water loss that occurs in sickle cell disease.
Figures




References
-
- Logsdon, N. J., Kang, J., Togo, J. A., Christian, E. P. & Aiyar, J. (1997) J. Biol. Chem. 272, 32723-32726. - PubMed
-
- Köhler, M., Hirschberg, B., Bond, C. T., Kinzie, J. M., Marrion, N. V., Maylie, J. & Adelman, J. P. (1996) Science 273, 1709-1714. - PubMed
-
- Warth, R., Hamm, K., Bleich, M., Kunzelmann, K., von Hahn, T., Schreiber, R., Ullrich, E., Mengel, M., Trautmann, N., Kindle P., et al. (1999) Pflügers Arch. 438, 437-444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

